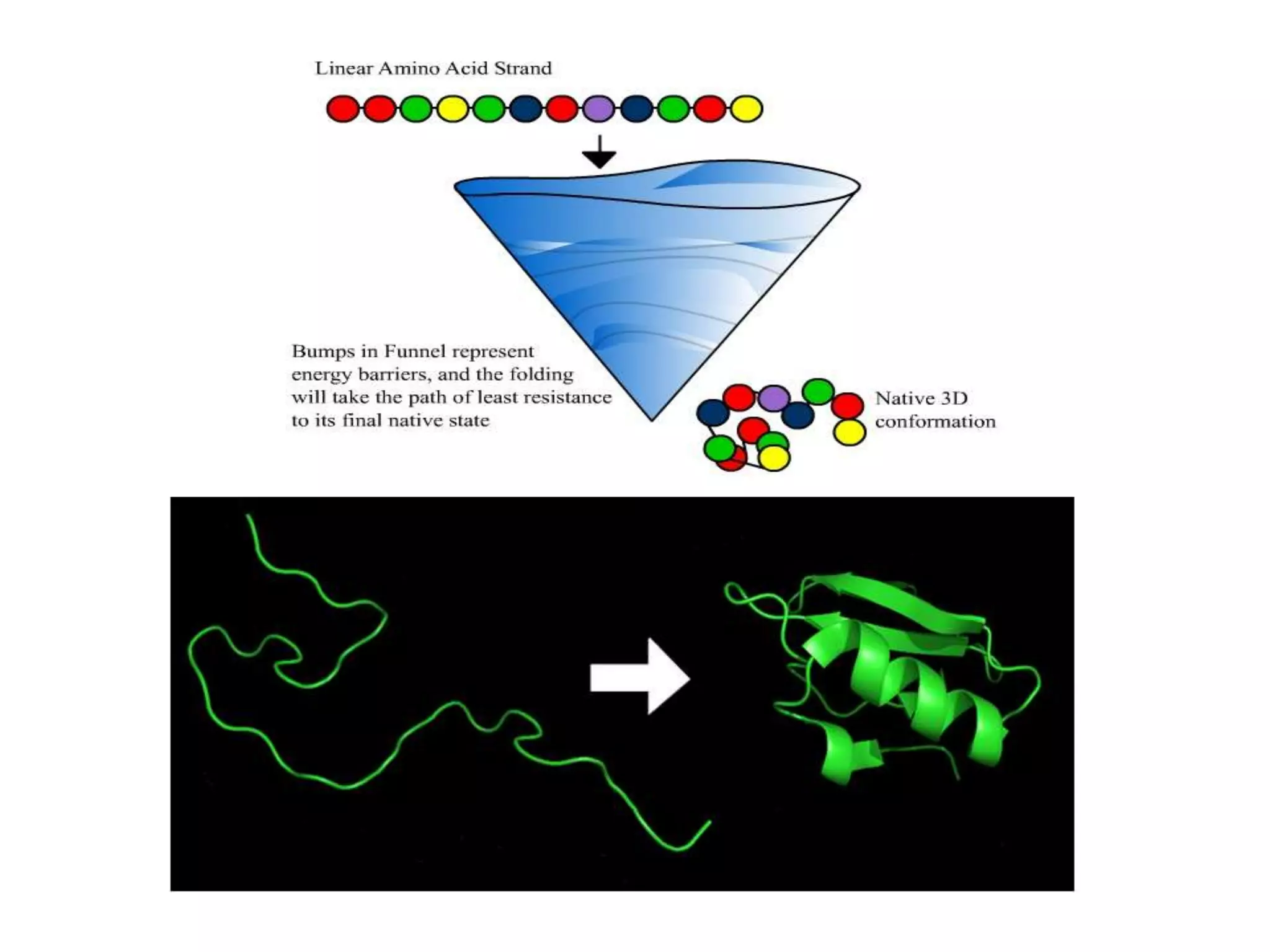
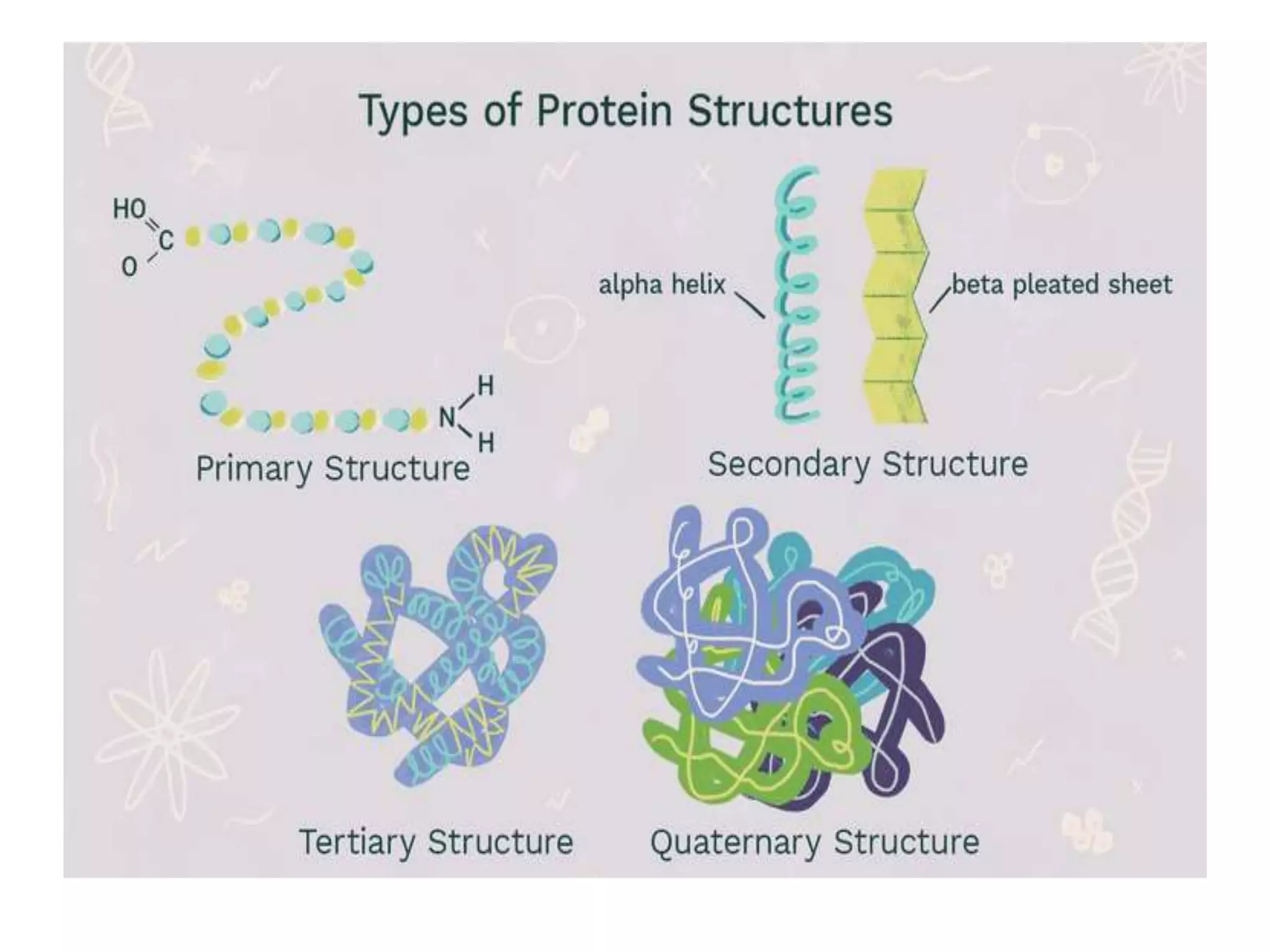
This document discusses protein structure and folding. It describes the four levels of protein structure: primary, secondary, tertiary, and quaternary. The primary structure is the amino acid sequence. Secondary structure involves folding into alpha helices or beta sheets. Tertiary structure is the overall 3D shape formed by interactions between amino acid side chains. Quaternary structure refers to interactions between multiple polypeptide chains in a protein. The document also discusses protein folding, denaturation, and misfolding, noting that many neurodegenerative diseases are associated with misfolded protein aggregates.