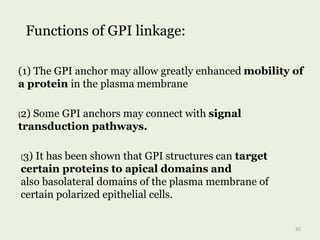
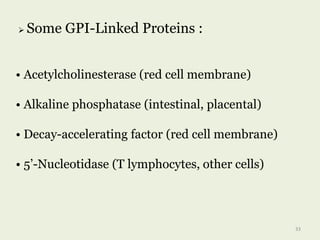
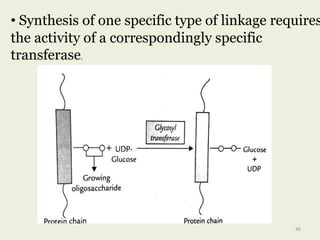
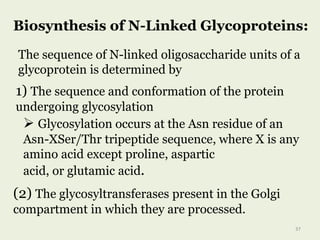
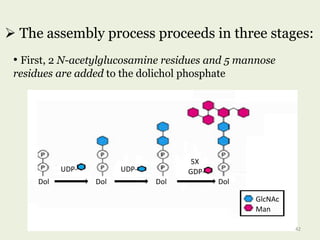
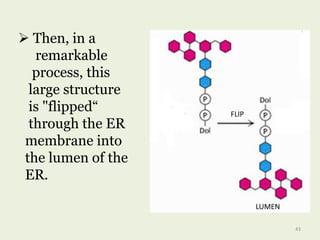
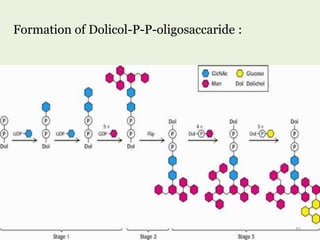
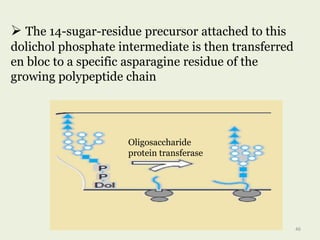
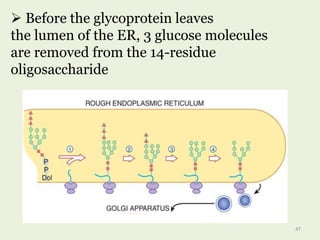
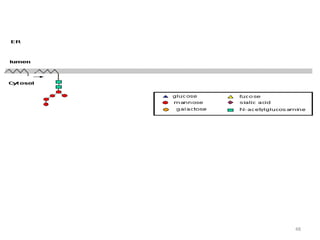
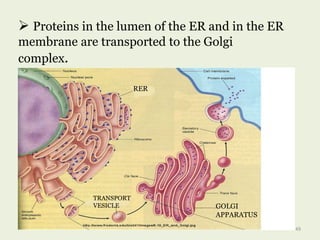
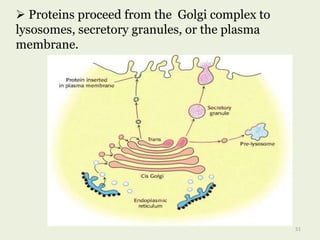
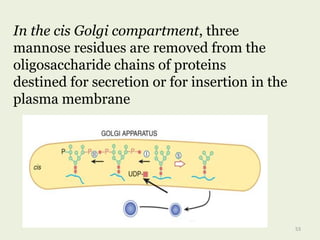
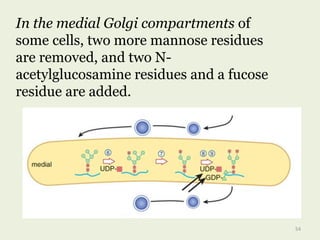
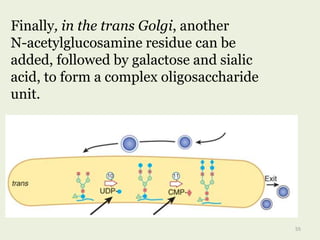
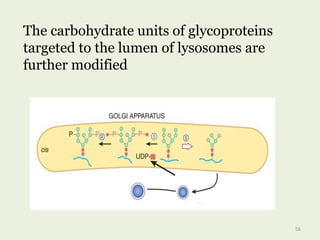
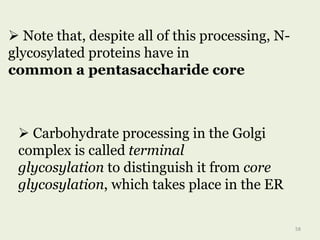
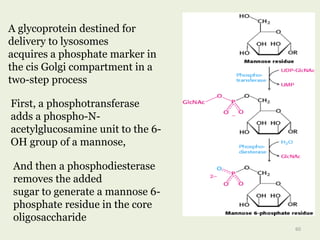
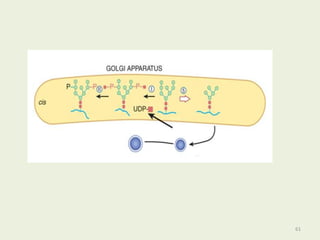
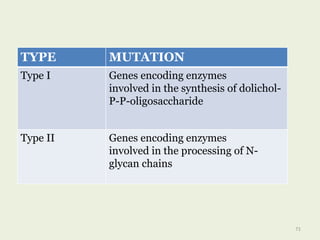
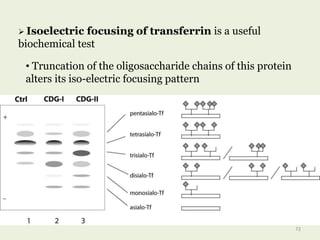
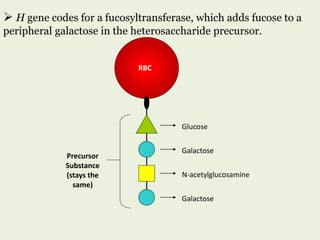
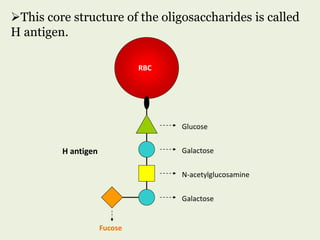
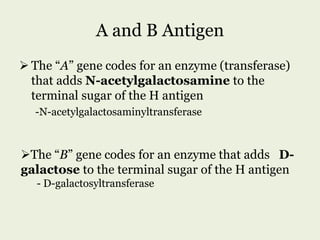
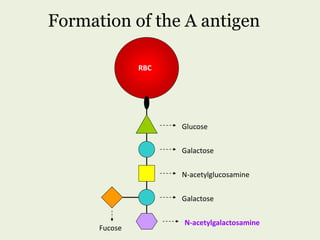
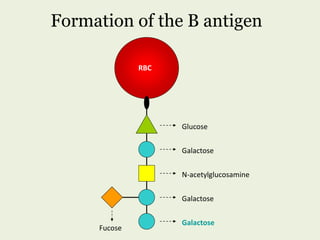
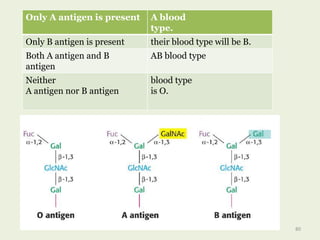
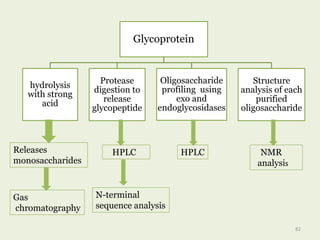
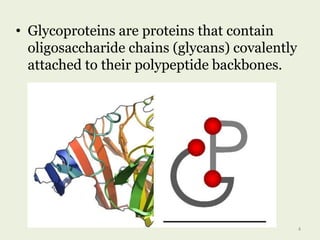
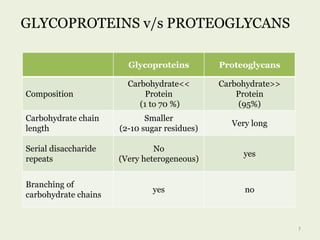
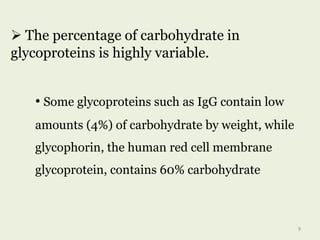
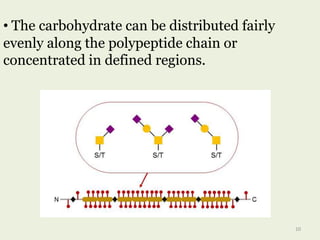
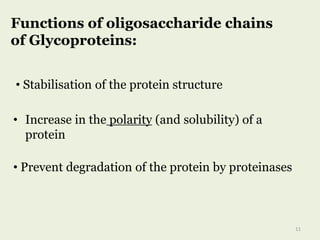
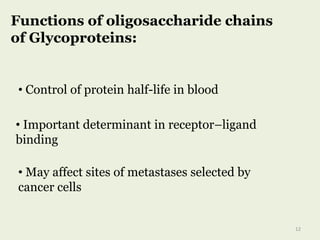
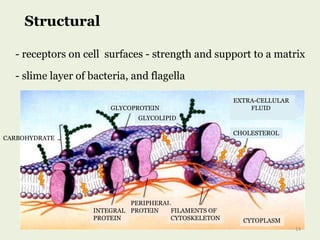
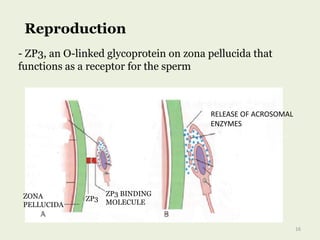
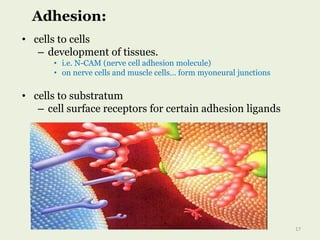
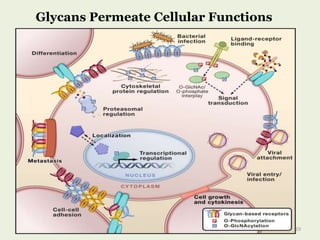
Glycoproteins are proteins that contain carbohydrate chains covalently attached. They can be O-linked, N-linked or GPI-anchored. Glycoproteins play important structural and functional roles like cell adhesion and acting as receptors. They are synthesized through a complex process in the endoplasmic reticulum and Golgi apparatus. Congenital disorders of glycosylation can occur from mutations affecting glycoprotein synthesis. Blood groups are also determined by glycoproteins on red blood cell surfaces.











![O-Linked
Glycoproteins
GalNAcSer(Thr)
linkage
Gal-Gal-Xyl-Ser
linkage
Galhydroxylysi
ne
GlcNAc-Ser[Thr]
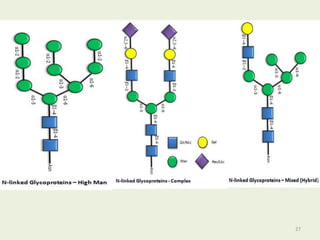
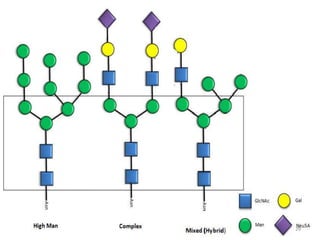
N-Linked
Glycoproteins
Complex
Hybrid
High-
mannose
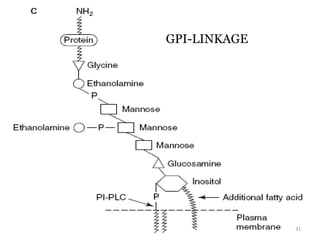
GPI-Linked
Glycoproteins
Other minor
groups
22](https://image.slidesharecdn.com/glycoproteins-170721145314/85/Glycoproteins-22-320.jpg)
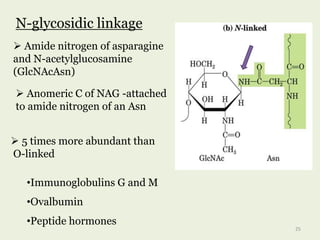
![•cell surface glycoproteins
•mucins
•viral glycoproteins
O-glycosidic linkage
Hydroxyl side chain of serine
or threonine and a sugar such
as Nacetylgalactosamine
(GalNAc-Ser[Thr])
Anomeric carbon of NAG …
attached to O of serine or
threonine
23](https://image.slidesharecdn.com/glycoproteins-170721145314/85/Glycoproteins-23-320.jpg)
![• Four subclasses of O-glycosidic linkages are
found in human glycoproteins :
O-glycosidic
linkages
GalNAcSer(
Thr)
Gal-Gal-Xyl-
Ser
Galhydroxyl
ysine
GlcNAc-
Ser[Thr]
•Predominant
linkage
24](https://image.slidesharecdn.com/glycoproteins-170721145314/85/Glycoproteins-24-320.jpg)