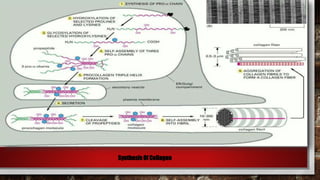
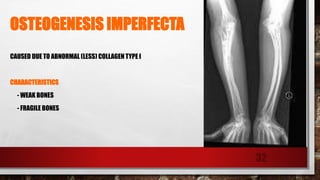
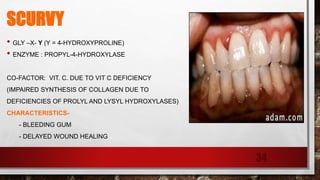
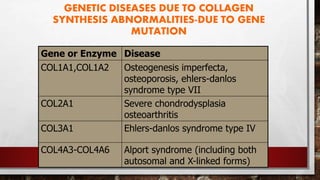
The document discusses fibrous proteins and collagen. It provides details on:
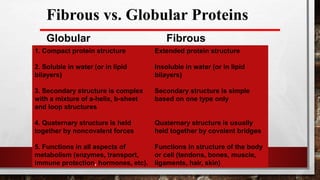
1) Fibrous proteins are usually insoluble and found in skin, connective tissue, blood vessels and other structures. They have high alpha-helix or beta-sheet content and examples include collagen, elastin, keratin and fibroin.
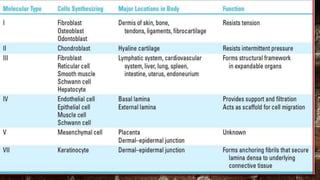
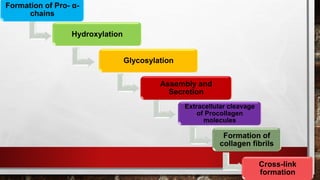
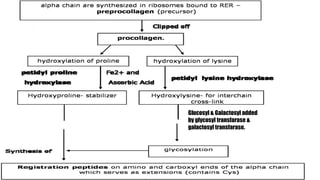
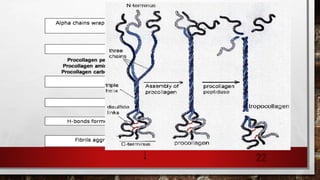
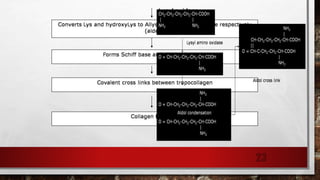
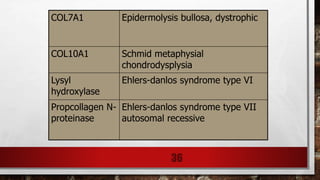
2) Collagen is the most abundant fibrous protein, making up 25-35% of the body's protein. It provides structure and strength, and there are at least 19 types designated by Roman numerals.
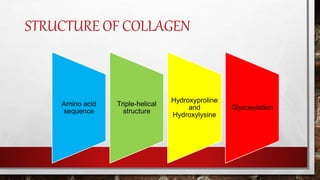
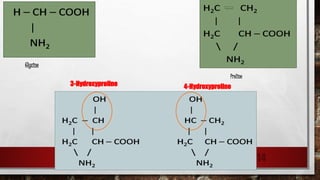
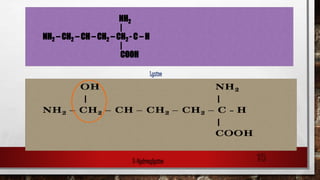
3) Collagen has a characteristic amino acid sequence of glycine-X-Y where X is often proline and Y can be hydroxyproline or hydroxy