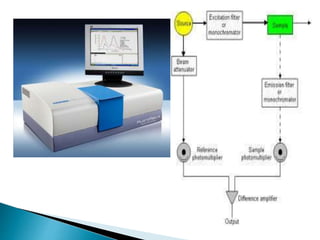
The document provides an overview of spectrofluorometry, detailing the principles of fluorescence and phosphorescence, including energy transitions, Stokes shift, and the role of solvent and temperature on fluorescence intensity. It discusses instrumentation for measuring fluorescence such as lasers and photomultipliers, and highlights various applications in detecting low-concentration materials like vitamins and hormones. Specific rules and effects that influence fluorescence outcomes, including the properties of different solvents and molecular structures, are also outlined.

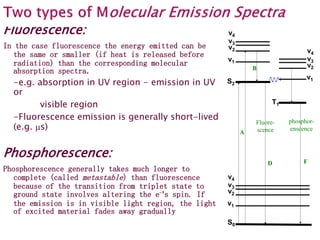
![An excited molecules can lose its
excess energy via several
processes
-Process B - Releasing E [energy] as heat
when changing within the same state
-The remaining energy can be release by
one of following Processes (C, D & E)
-Process C - Transfer its remaining E to
other chemical species by collision
-Process D - Emitting photons when
falling back to the ground state -
Fluorescence
-Process E1 - Undergoing internal
transition within the same mode of the
excited state
-Process E2 - Undergoing intersystem
crossing to a triplet sublevel of the
excited state
-Process F - Radiating E from triplet to
ground state (triplet quenching) -
Phosphorescence
S0
T1
S2
S1
v1
v2
v3
v4
v1
v2
v3
v4
v1
v2
v3
v4
v1
v2
v3
v4
Inter-
system
crossing
Internal
transition
B
B
E1
E2
C
F
A
B
Fluorescence
D
Fluorescence
Jablonsky diagram
Phosphorescence](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-3-320.jpg)
![Review: Fate of Excited State
When the molecule absorb a photon, it will be excited to
an upper electronic and upper vibrational state. Three
things can happen while it is in this excited state:
1- It may undergo a radiation less loss [vibrational relaxation]
of electronic energy through collision & other
interactions. Most molecules do not fluoresce because
of this deactivation process.
2-It can emit a photon & Fluoresce
3-It can undergo a transition to a metastable state [triplet]
and phosphoresce](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-4-320.jpg)
![Fluorescence
1- Fluorescence is and Emission phenomenon: the energy
transition from a higher to lower state within the molecule
that is being measured by the detection of emitted radiation.
[not the absorption as in Spectrophotometry]
2-It includes:
a] Excitation
[due to input of energy by absorption of EMR-higher energy/Shorter λ]
b] Emission
[ resulted into fluorescence with lower energy/Longer λ]
3-The difference between these two λ is known as the
“STOKES SHIFT” [Larger the shift better the results]](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-5-320.jpg)
![Intensity of Fluoresecnce
-Intensity is proportional to the:
1-Ground-state population of Fluorophore
2-The rate of absorption
3-The volume of sample illuminated
4-The two λ [excitation & emission]](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-7-320.jpg)
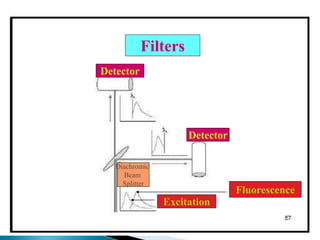
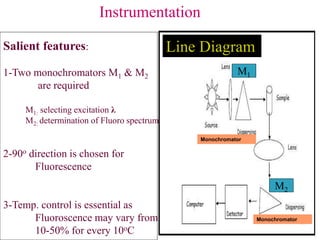
![Instrumentation
--Sources:
LASER, Deuterium, Halogen,
Xenon Lamp [150W, 300-1300nm]
--Dispersing
Filter, Monochromator, Prism
--Detectors:
Photomultiplier,
Charge Coupled Device](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-9-320.jpg)
![Rules for Fluorescence
1-Not observed from saturated hydrocarbons as there are no or n electrons.
Weak fluorescence observed sometimes in the vacuum UV.
2-Rarely observed from non-aromatic hydrocarbons that have some
double bonds.
3-Phopsphorescence is often favourable in aromatic molecules containing
corbonyl groups.
4-Substituents attached to aromatic rings influence Fluorescence. These groups
change the nature of lowest-lying excited state [S1]
5-Addition of rings increases the Fluorescence yield
6-Aliphatic molecules tend to photodissocate rather than fluoresce](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-11-320.jpg)

![APPLICATIONS
-The LASER [Light Amplification by Stimulated Emission of Radiation]
induced fluorescence (LIF) is very sensitive for scanning single
molecule.
-The detection of Non-Fluorecence compounds may be achieved by
coupling Flour Probe [extrinsic Fluorescence].
E.g.: Amino acids:Dansyl chloride Peptides: 0-pthaladehyde
Nucleotide strands by Acridinne [difference in Stokes Shift]
Single strand-Red colour Double strand-Green colour
-Major use for quantitative determination of materials present in
concentrations too low such as Vitamin in food stuffs, NADH,
Hormones, Carcinogenes, Cholestrol, Porphyrins etc.](https://image.slidesharecdn.com/spectrofluorometry-221018050805-8d163046/85/Spectrofluorometry-ppt-14-320.jpg)