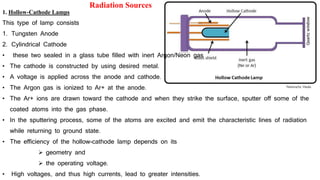
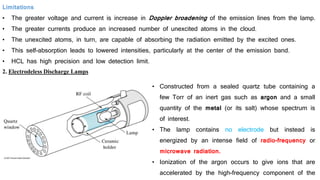
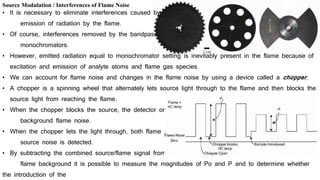
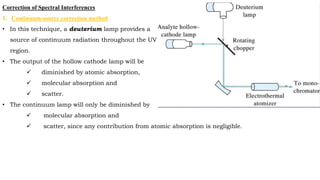
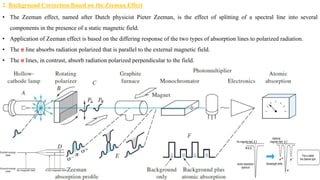
This document discusses light sources and background corrections for Atomic Absorption Spectroscopy (AAS). It describes two main light sources: hollow-cathode lamps and electrodeless discharge lamps. Hollow-cathode lamps consist of a tungsten anode and metal cathode enclosed in a glass tube with inert gas. An applied voltage excites the gas to produce characteristic radiation from the coated metal. Electrodeless discharge lamps contain inert gas and metal salt excited by radio waves. The document also discusses methods to correct for spectral interferences, including continuum source correction, Zeeman effect background correction, and source self-reversal using high and low lamp currents.