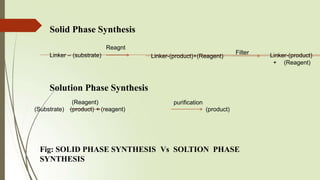
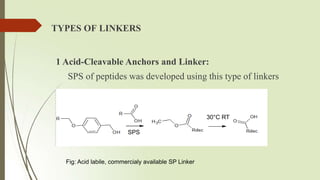
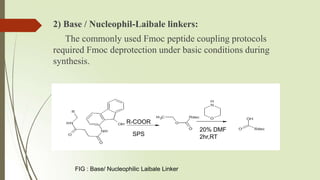
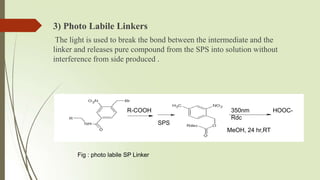
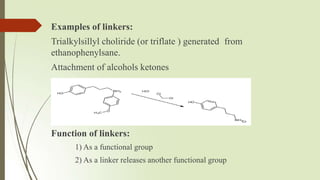
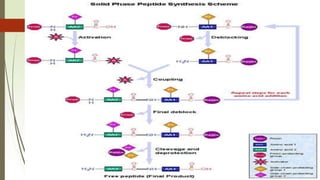
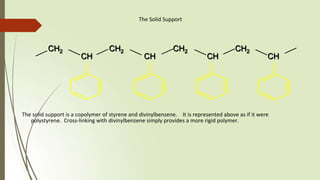
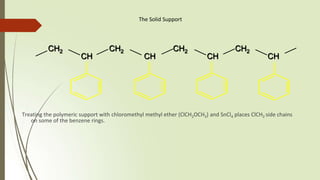
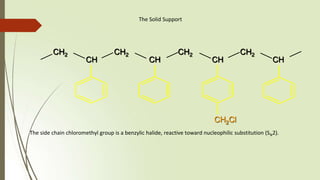
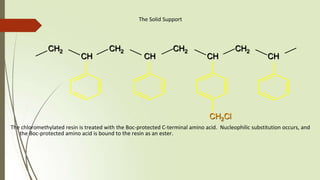
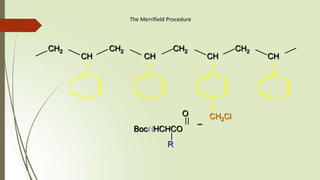
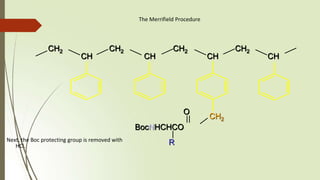
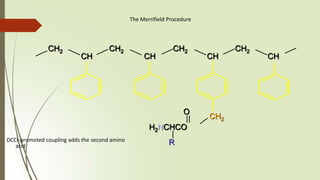
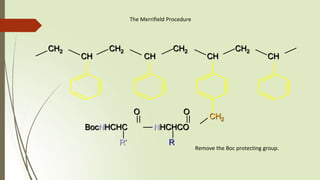
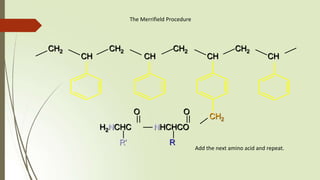
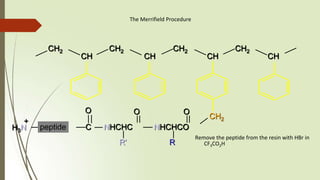
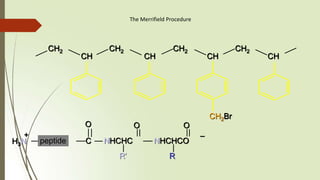
The document provides an overview of solid phase synthesis. It describes how solid phase synthesis involves coupling reagents to an inert solid support to perform multi-step organic synthesis. The key steps include attaching the starting material to a resin via a linker, performing sequential reactions on the bound intermediate, then cleaving the final product from the resin. The Merrifield method from 1963 pioneered this technique by automating the synthesis of peptides on an insoluble polystyrene resin, enabling efficient purification and the potential for parallel reactions.