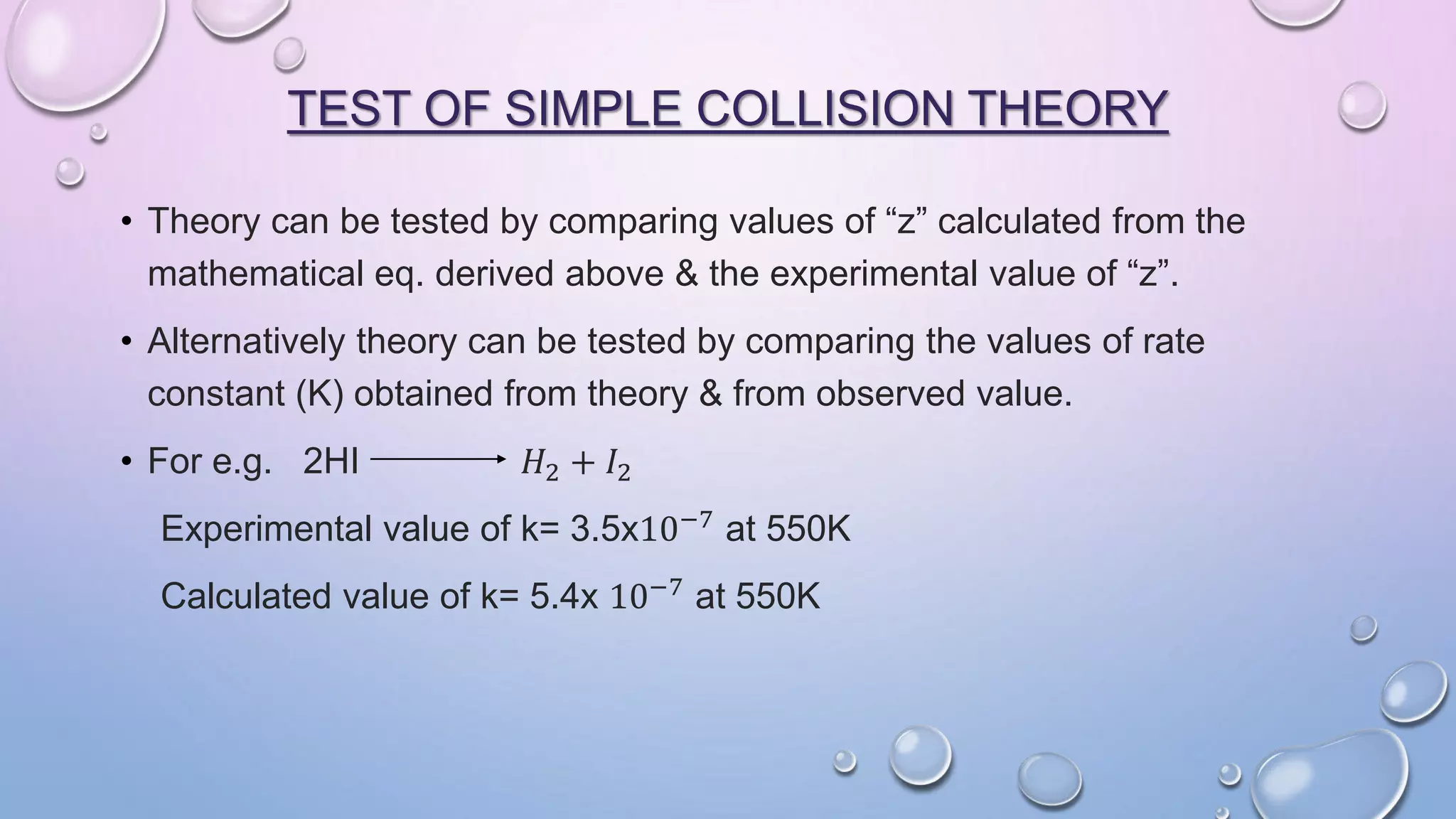
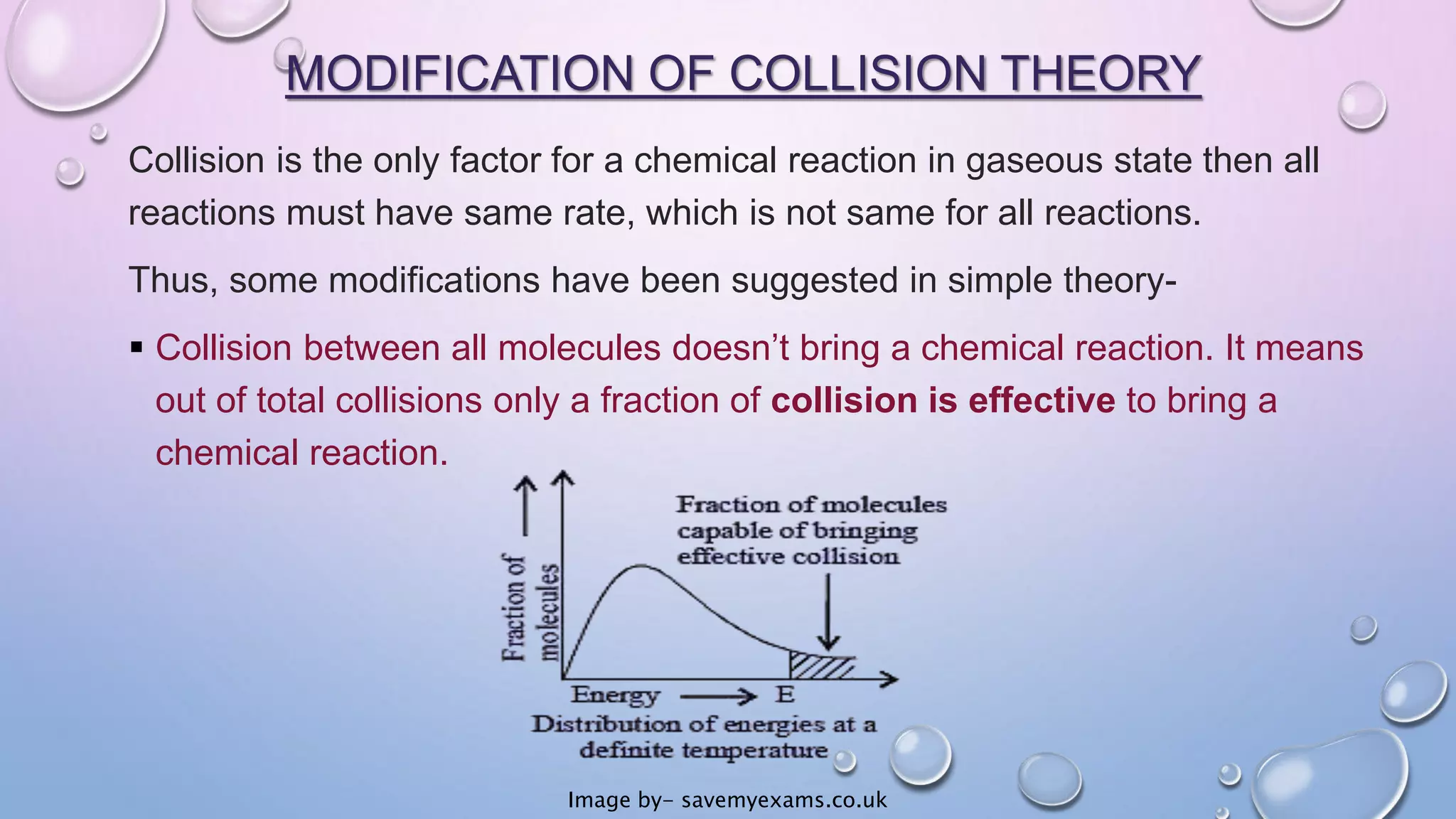
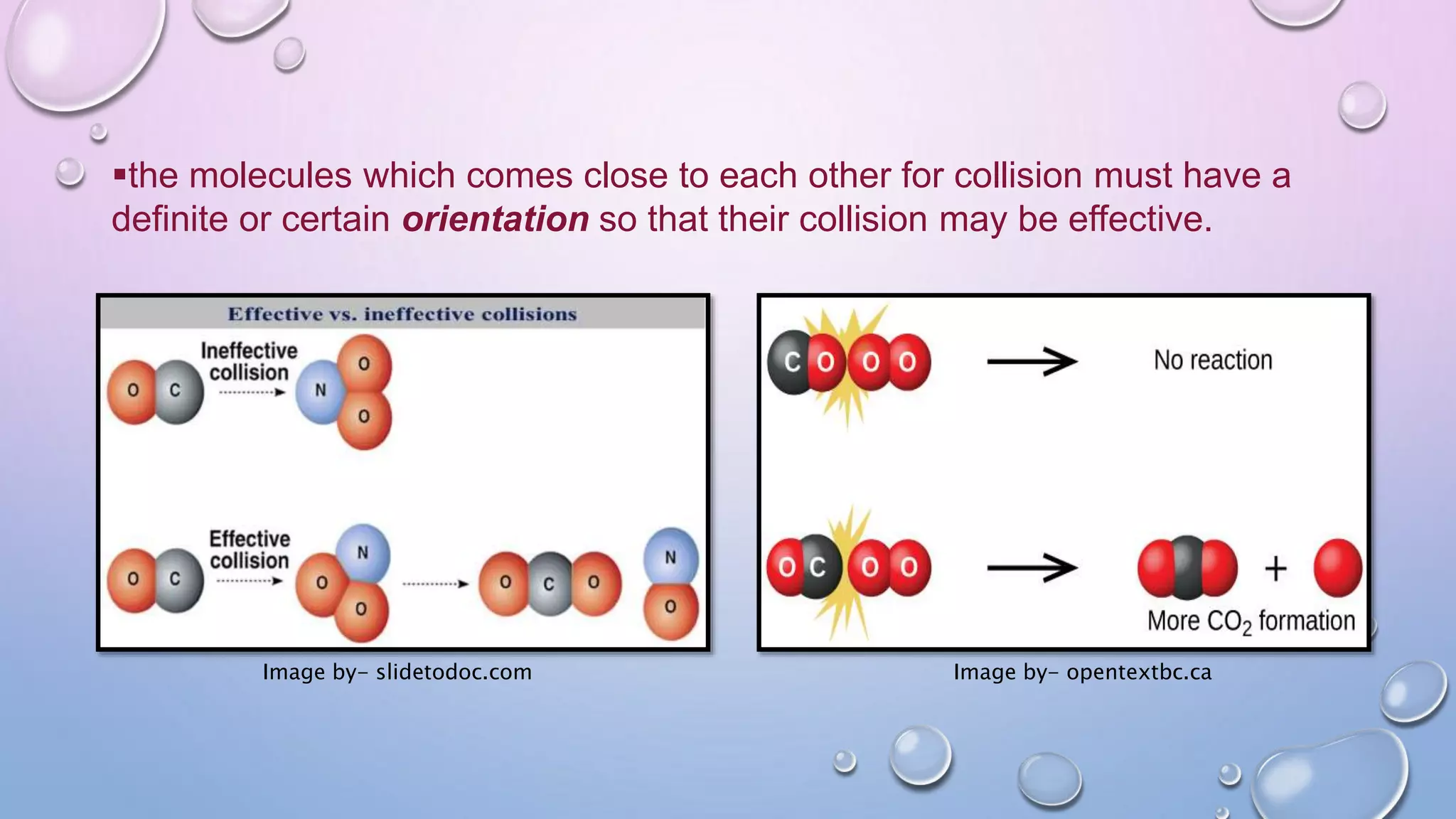
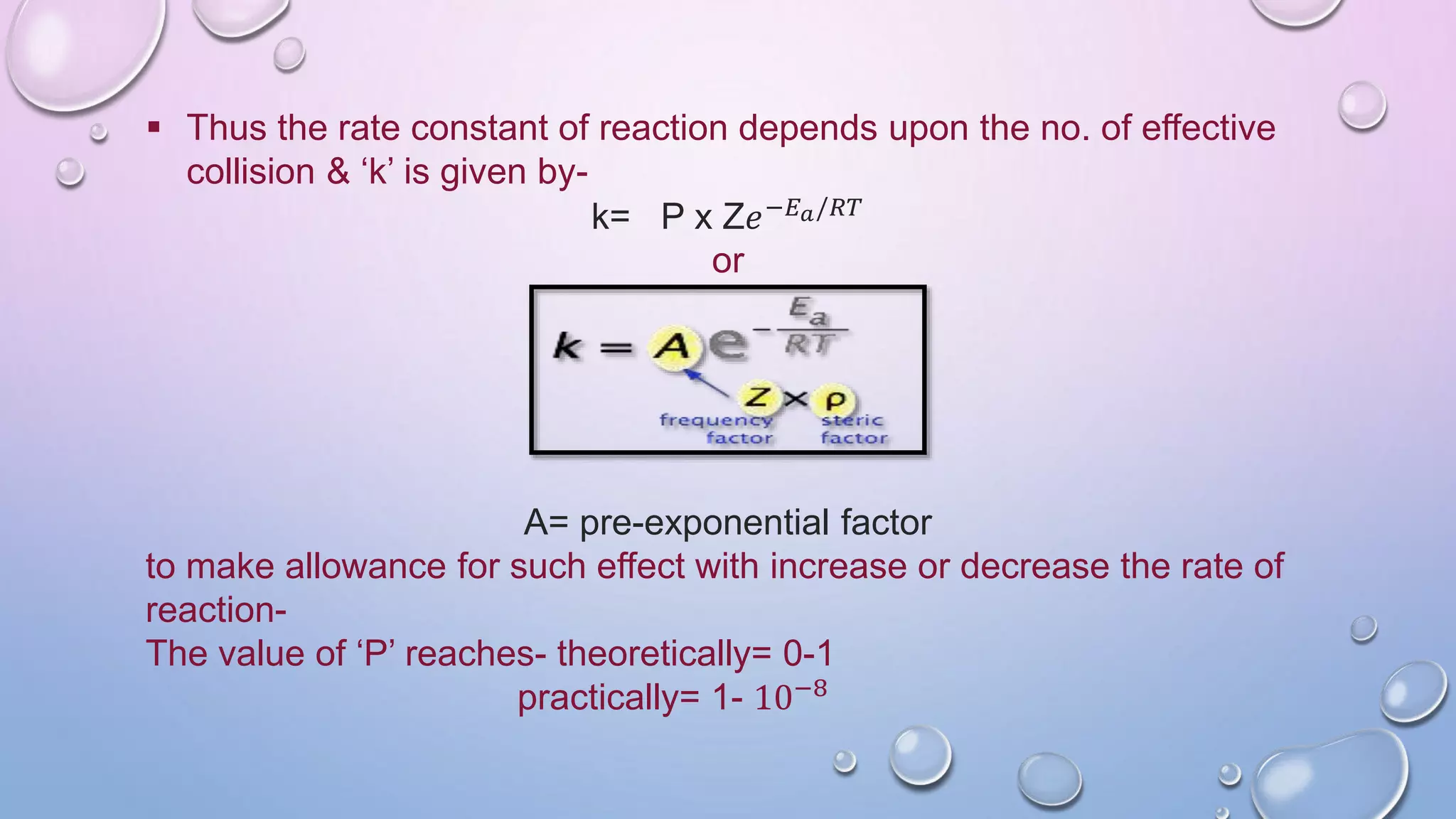
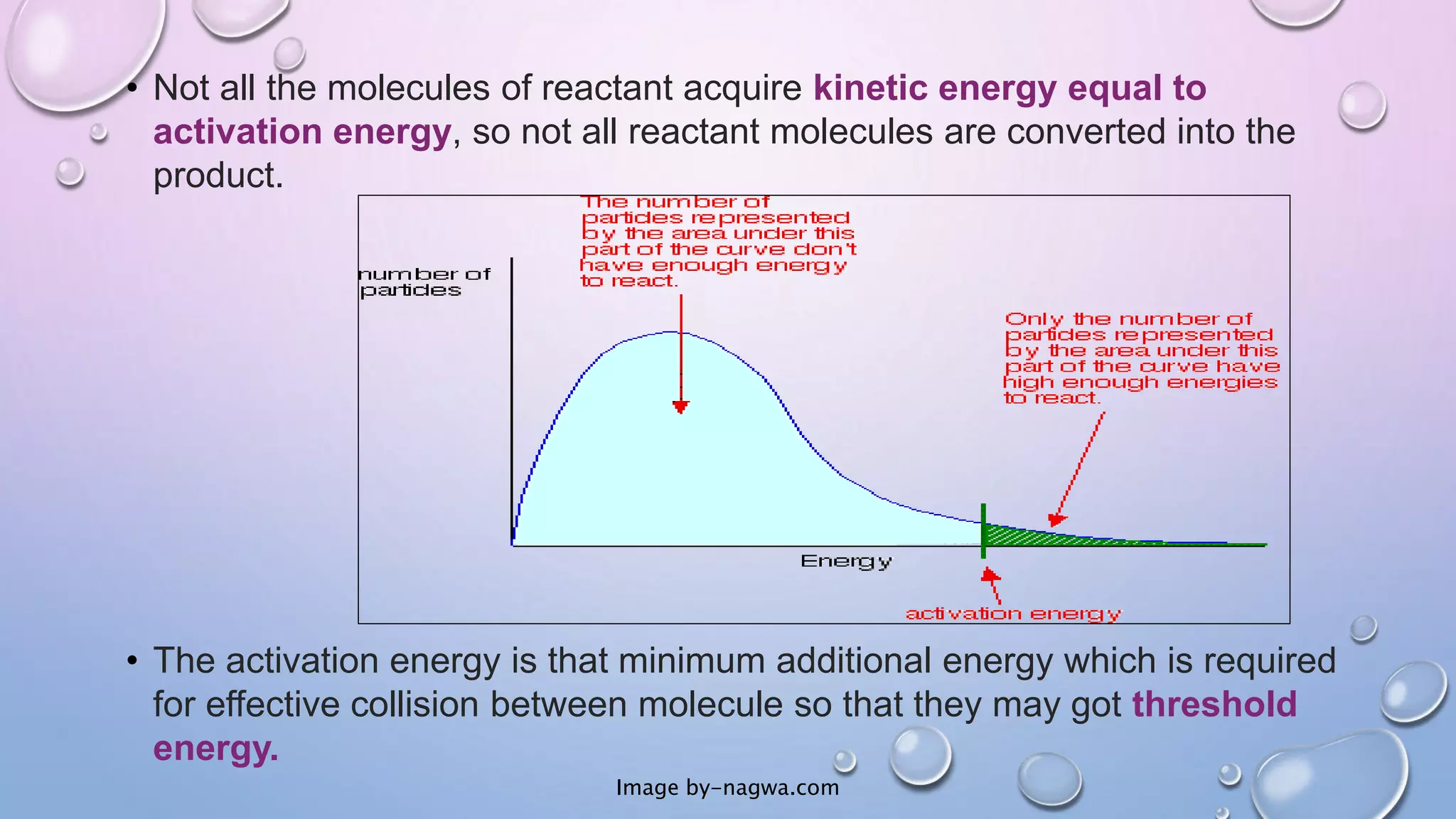
Collision theory proposes that for a chemical reaction to occur, reactant molecules must collide with sufficient energy to overcome an activation energy barrier. The rate of reaction is directly proportional to the collision frequency and a fraction of effective collisions. However, collision theory fails to explain several observed reaction rates and does not account for molecular orientations or internal motions. Modifications were made to incorporate effective collision probability but correlations between this factor and reaction characteristics remain unclear.




![MATHEMATICAL TREATMENT OF COLLISION THEORY
A+B Product
According to collision theory,
Rate of reaction ∝ Number of collision between A&B
According to Rate law, Rate of reaction ∝ 𝑨 [𝑩]
So, Number of collision ∝ concentration of reactants
According to Arrhenius law, Rate of reaction= Z𝑒−𝐸𝑎/𝑅𝑇 …(1)
Where, Z= no. of binary collision per unit time, 𝐸𝑎= activation energy
According to kinetic theory, the rate of reaction is related with the no. of
molecule per unit volume i.e. rate ∝ nxn](https://image.slidesharecdn.com/dorqkvgjq0xueyvqgycs-collision-theory-sem-1-jyotsana-panwar-230201074544-aba8e5f5/75/Collision-Theory-7-2048.jpg)

![k= 4𝑛2𝜎2 𝜋𝑅𝑇
𝑚
x 𝑒−𝐸𝑎/𝑅𝑇 /𝑛2
After simplifying we’ll get,
k= 4𝜎2 𝜋𝑅𝑇
𝑚
x 𝑒−𝐸𝑎/𝑅𝑇
…(5)
k= z𝑒−𝐸𝑎/𝑅𝑇 , where z= 4𝜎2 𝜋𝑅𝑇
𝑚
is the collision number.
Collision number / collision frequency is the number of collisions per unit
time in per unit volume.
When two molecules of different types are involved in collision, z becomes-
z= 𝜎2[8𝜋𝑅𝑇 (
𝑚1+𝑚2
𝑚1𝑚2
] where, (
𝑚1+𝑚2
𝑚1𝑚2
)= reduced mass](https://image.slidesharecdn.com/dorqkvgjq0xueyvqgycs-collision-theory-sem-1-jyotsana-panwar-230201074544-aba8e5f5/75/Collision-Theory-9-2048.jpg)