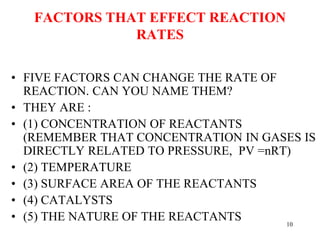
The document explains reaction rates, emphasizing that rates are the amount of substance divided by time, often measured in moles per second or molarity per second. It details how factors such as concentration, temperature, surface area, and the presence of catalysts affect these rates, alongside the collision theory which states reactions occur upon molecular collisions. Additionally, it discusses the reaction order, demonstrating how to determine it through the method of initial rates with experimental data.










![HOW CONCENTRATION EFFECTS
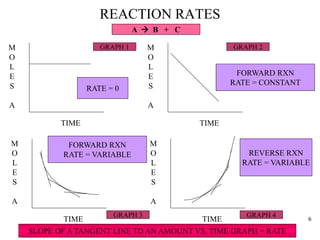
REACTION RATES (THE RATE EQUATON)
• THEREFORE OUR EQUATION MAY BE WRITTEN AS:
RATE = CONSTANT x CONCENTRATIN RAISED TO
SOME POWER OR
RATE = k x [A]n
• k = A CONSTANT CALLED THE SPECIFIC RATE
CONSTANT (IT IS CONSTANT FOR A SPECIFIC
REACTION AT A SPECIFIC TEMPERATURE)
• [A] = THE CONCENTRATION OF REACTANT A IN
MOLES PER LITER (BRACKETS MEAN IN MOLES
PER LITER)
• n = THE POWER TO WHICH CONCENTRATION
MUST BE RAISED (ALSO CALLED REACTION
ORDER)
16](https://image.slidesharecdn.com/rates1-100210114522-phpapp01/85/Reaction-Rates-16-320.jpg)


![HOW CONCENTRATION EFFECTS
REACTION RATES (INITIAL RATES)
• USING THE METHOD OF INITIAL RATES REQUIRES
THAT A REACTION BE RUN AT SERIES OF DIFFERENT
STARTING CONCENTRATIONS AND THE RATE BE
DETERMINED FOR EACH.
• GIVEN THE FOLLOWING DATA FOR THE REACTION
A B + C
(TABLE 1)
• EXPT [A] RATE (M/SEC)
1 1 x 10 -3 4 x 10 -1
2 2 x 10 -3 8 x 10 -1
3 4 x 10 -3 16 x 10 -1
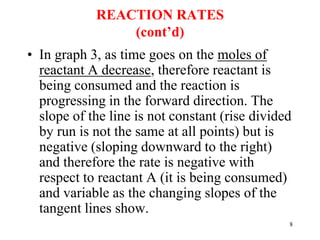
• AS CONCENTRATION OF A DOUBLES, RATE DOUBLES.
THE REACTION IS FIRST ORDER IN REACTANT A
• RATE = k[A]1 OR RATE = k[A] 19](https://image.slidesharecdn.com/rates1-100210114522-phpapp01/85/Reaction-Rates-19-320.jpg)
![HOW CONCENTRATION EFFECTS
REACTION RATES (INITIAL RATES)
• FOR THE REACTION: A + B C + D
• (TABLE 2)
1 1 x 10 -3 1 x 10 –3 4 x 10 -1
2 2 x 10 -3 1 x 10 -3 8 x 10 -1
3 1 x 10 -3 2 x 10 -3 16 x 10 –1
• USING EXPT 1 AND 2, [A] DOUBLES AND [B] IS
CONSTANT. THE DOUBLING OF THE RATE IS
THEREFORE CAUSED BY REACTANT A AND THE ORDER
WITH RESPECT TO A IS FIRST.
• USING EXPT 1 AND 3, [A] IS CONSTANT AND [B] IS
DOUBLED. THE FOUR TIMES RATE INCREASE IS
THEREFORE CAUSED BY REACTANT B AND THE ORDER
WITH RESPECT TO B IS SECOND.
• RATE = k[A]1[B]2 OR RATE = k[A][B]2
20](https://image.slidesharecdn.com/rates1-100210114522-phpapp01/85/Reaction-Rates-20-320.jpg)
