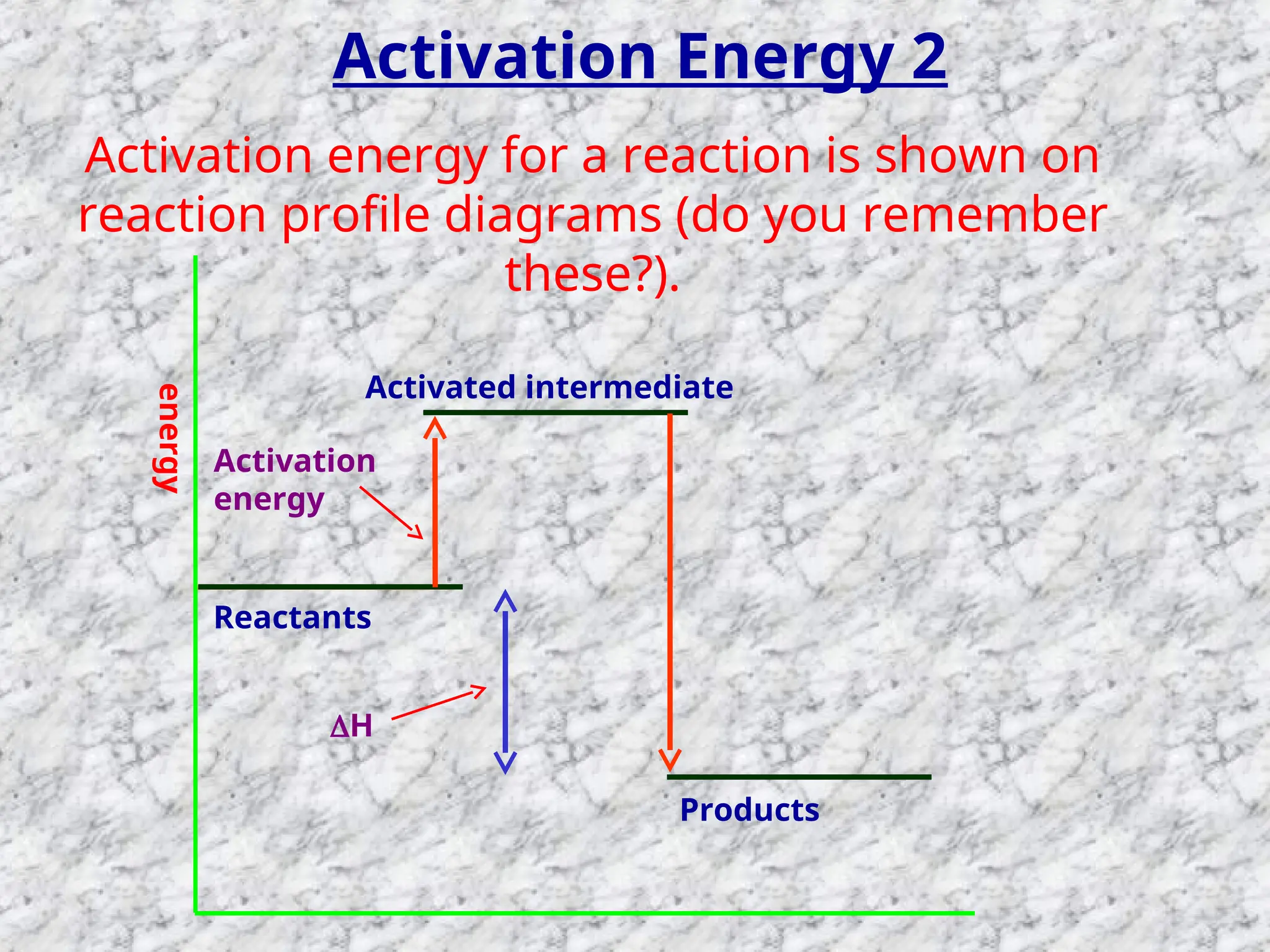
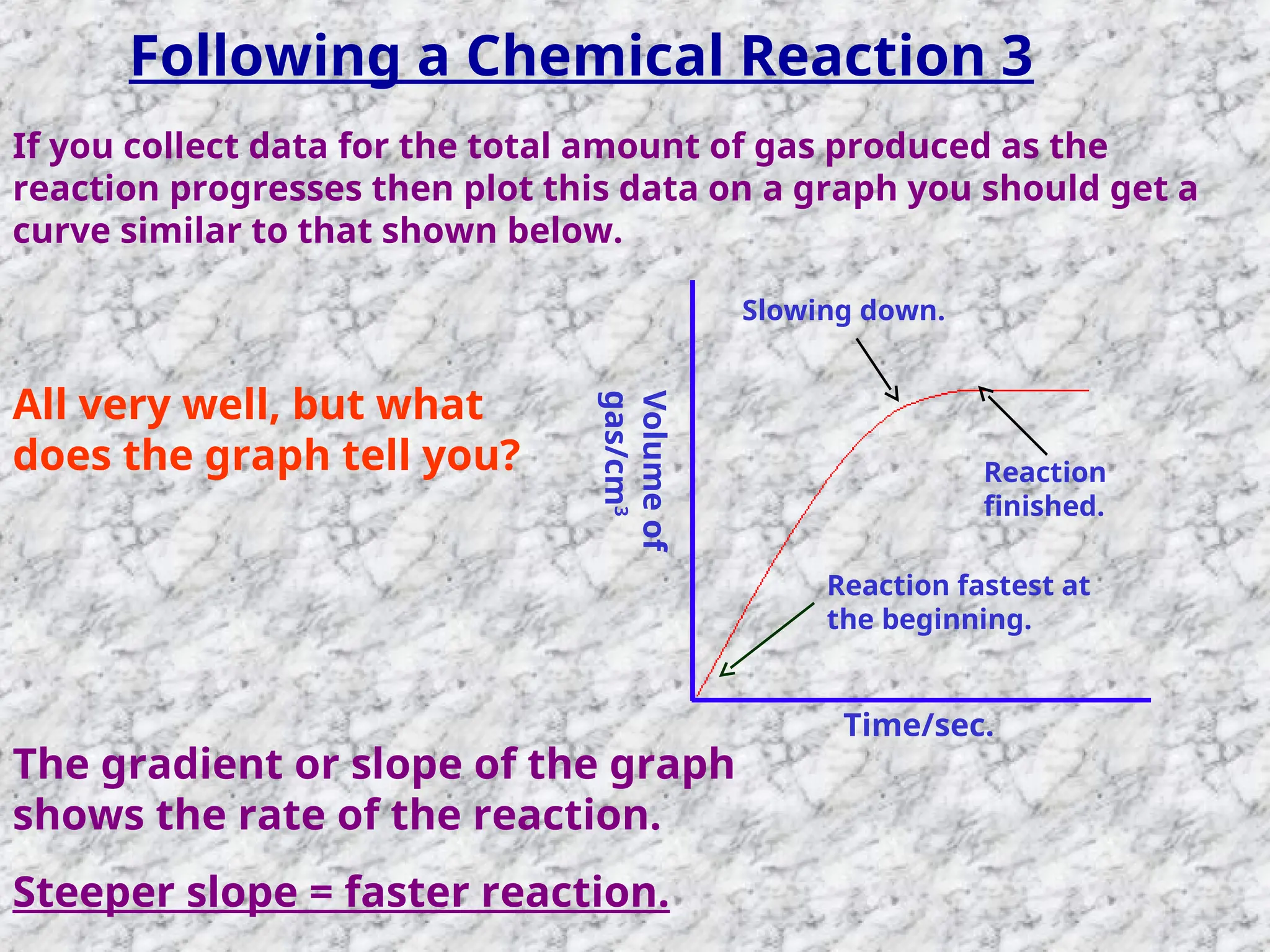
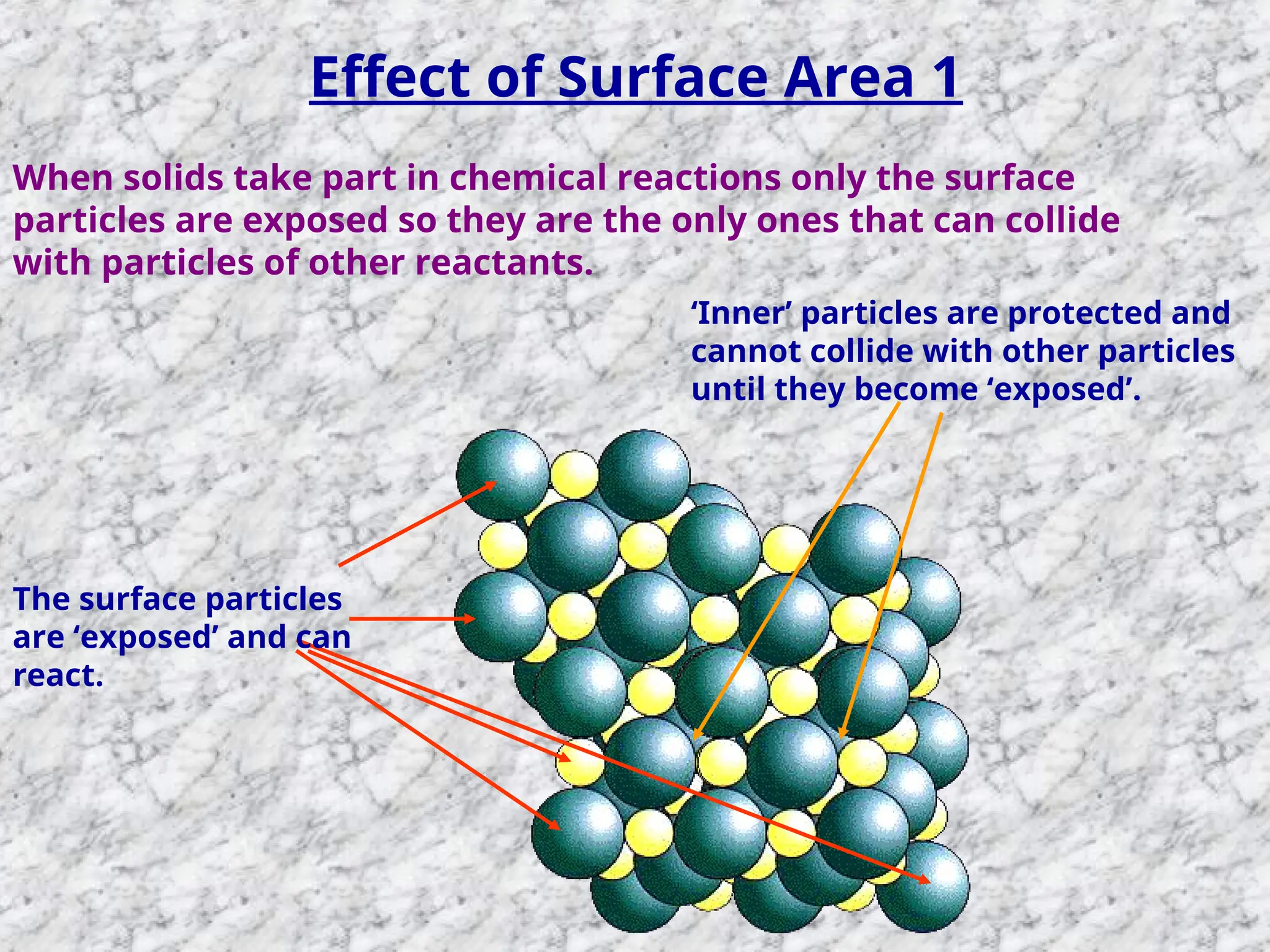
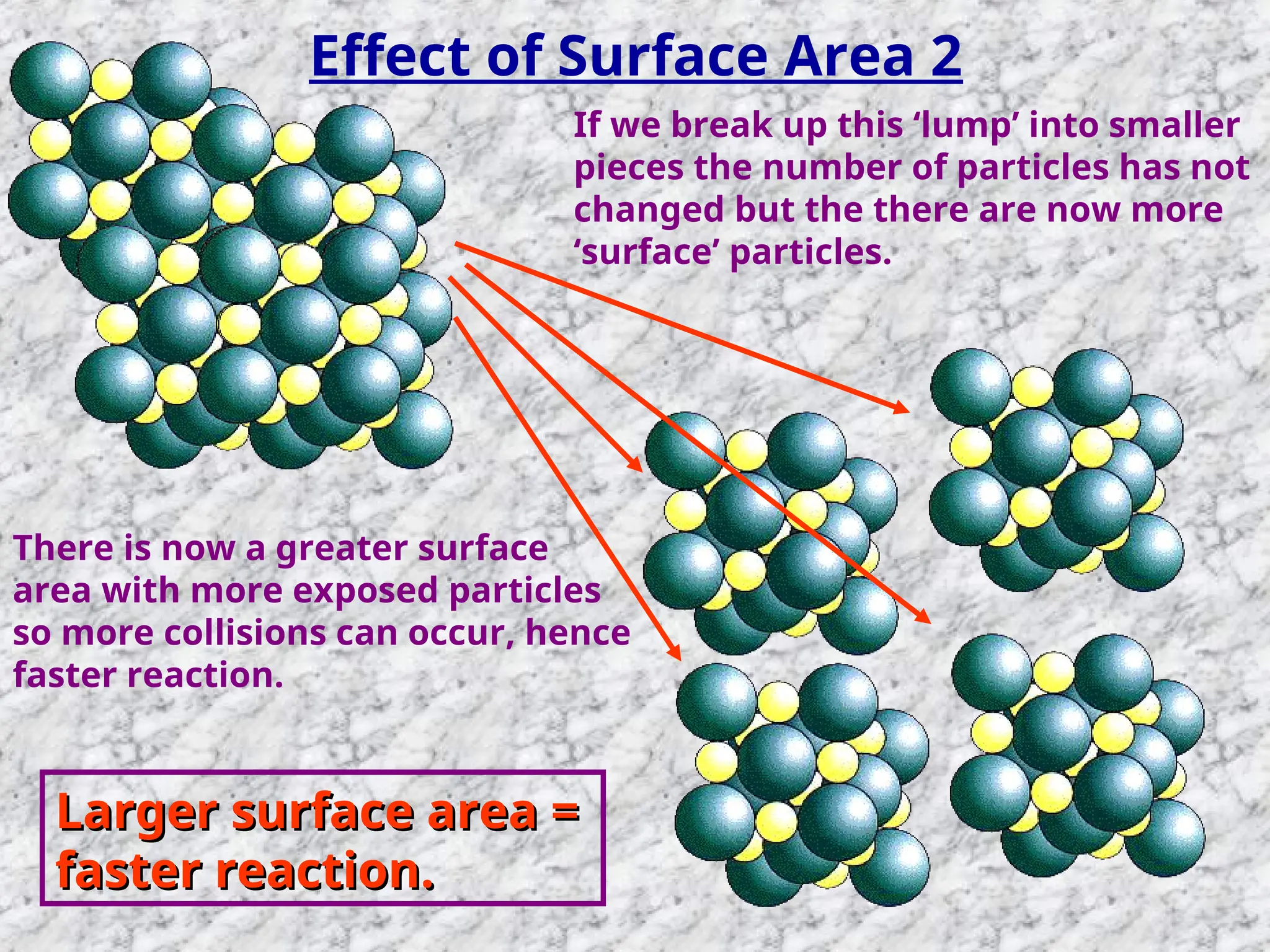
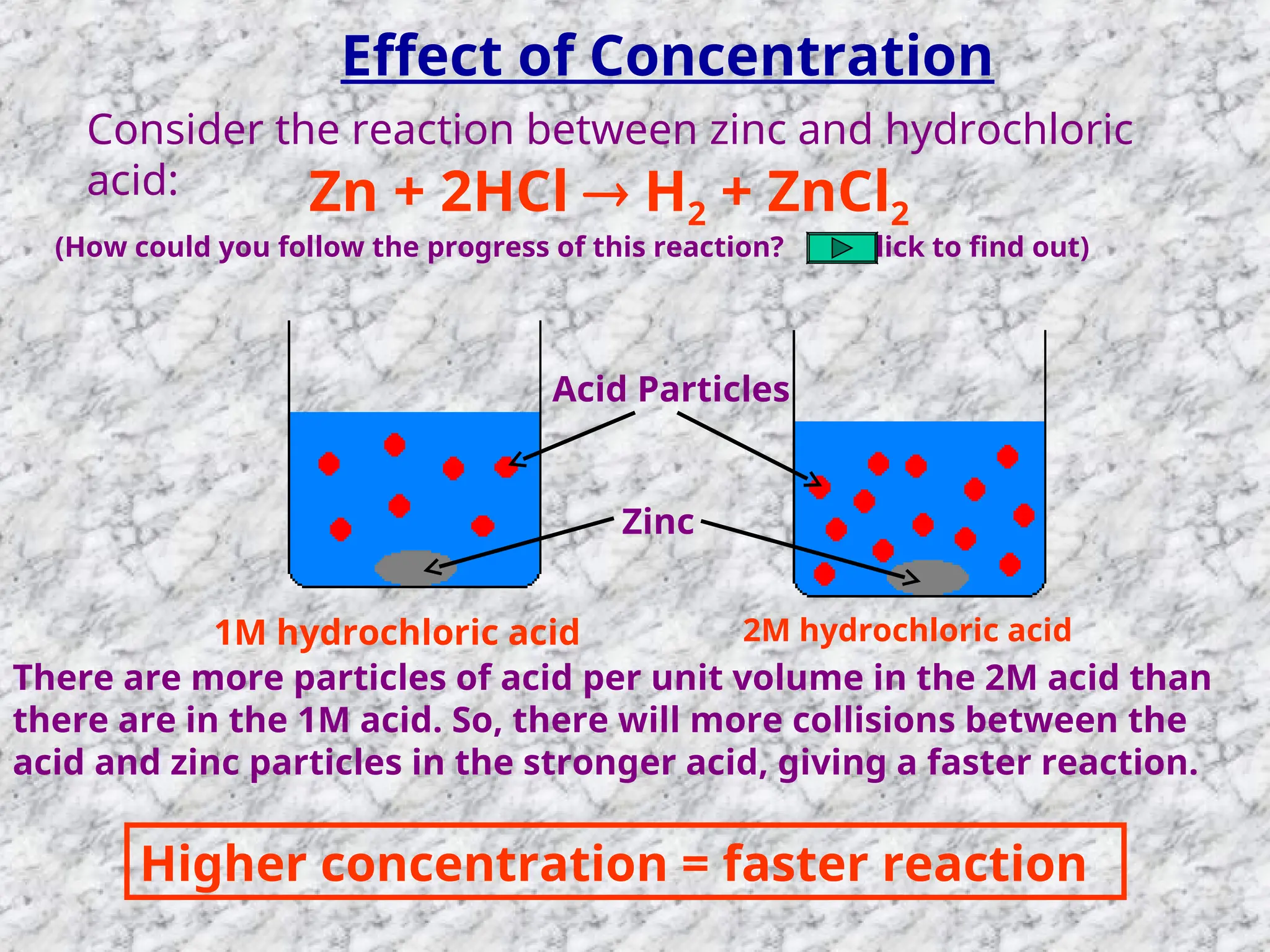
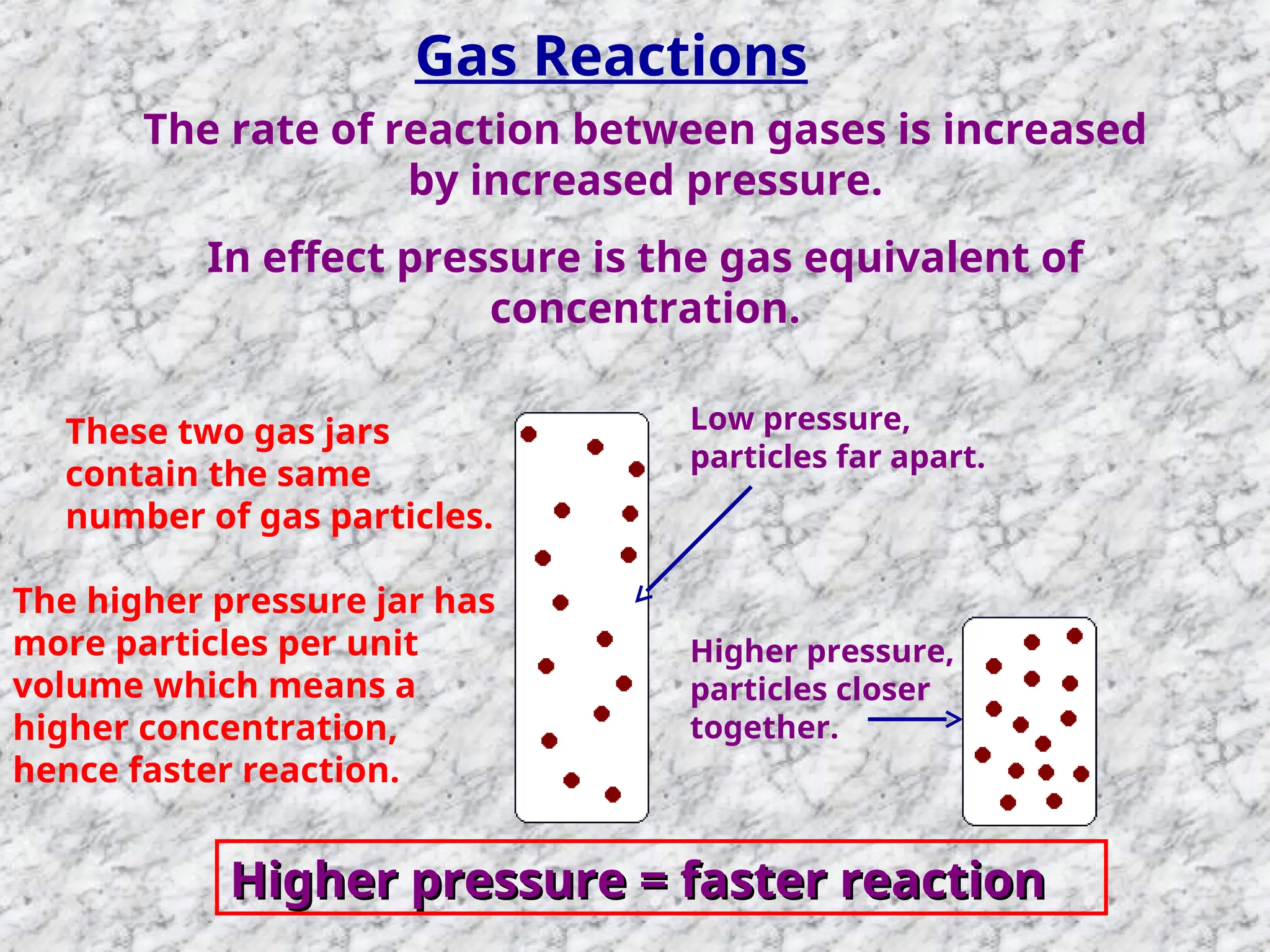
The document outlines factors affecting the rate of chemical reactions, emphasizing the need for reactant particles to collide with sufficient energy to overcome activation energy and form new bonds. Key factors for increasing reaction rates include increasing surface area, concentration, temperature, and using catalysts. The presentation serves as a refresher for A-level chemistry students, explaining how to measure reaction progress and the effects of various parameters on reaction rates.