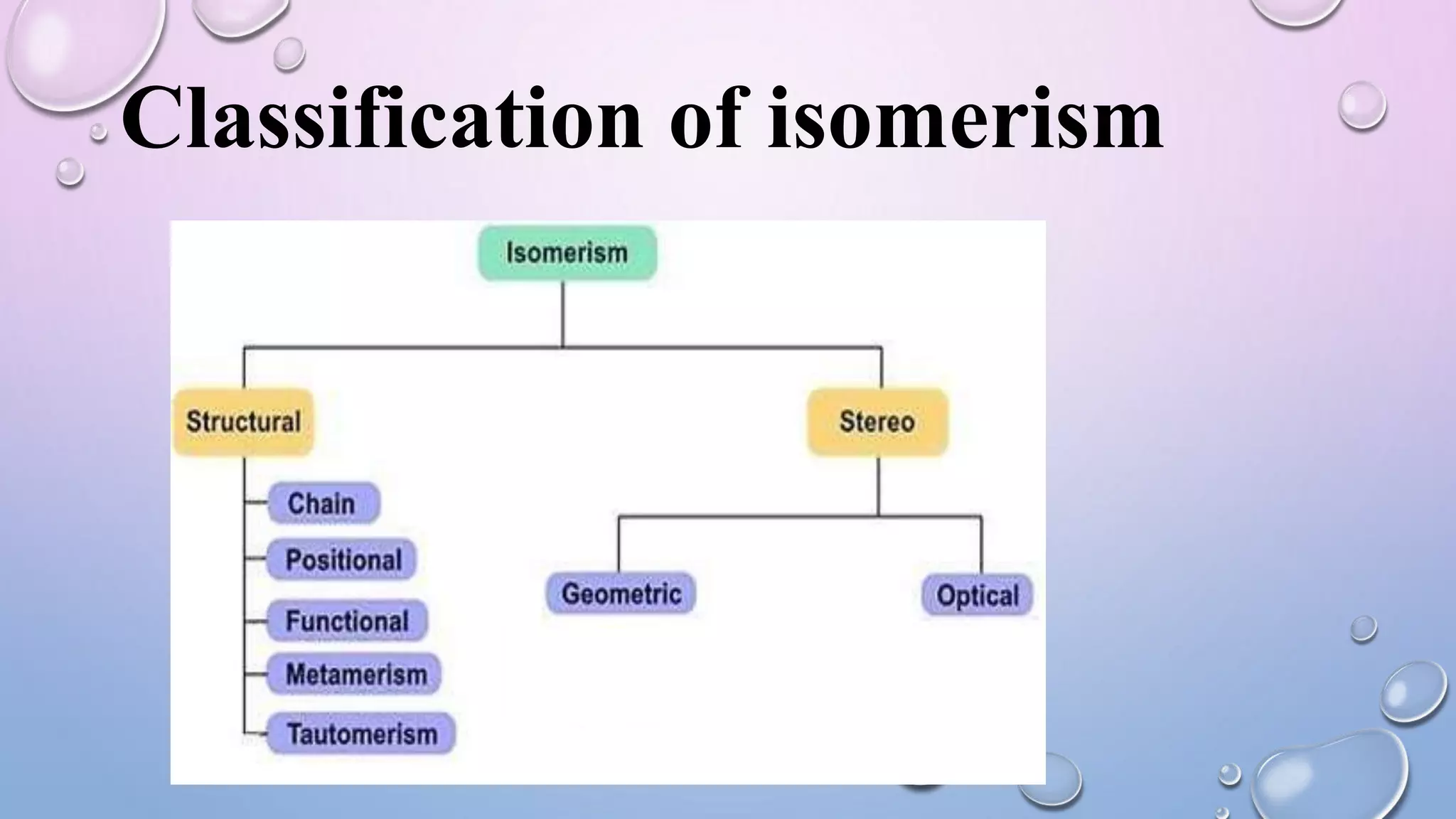
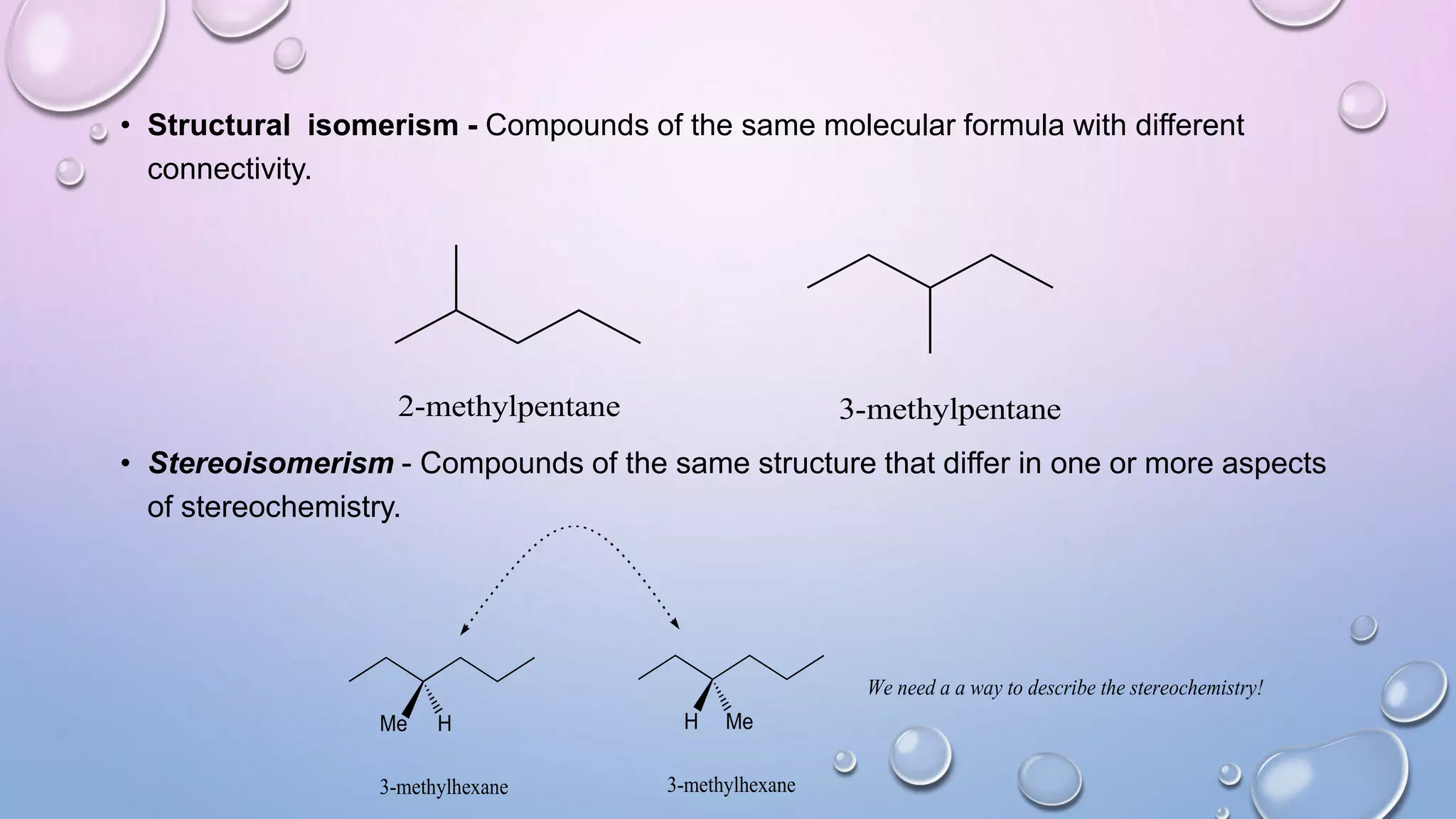
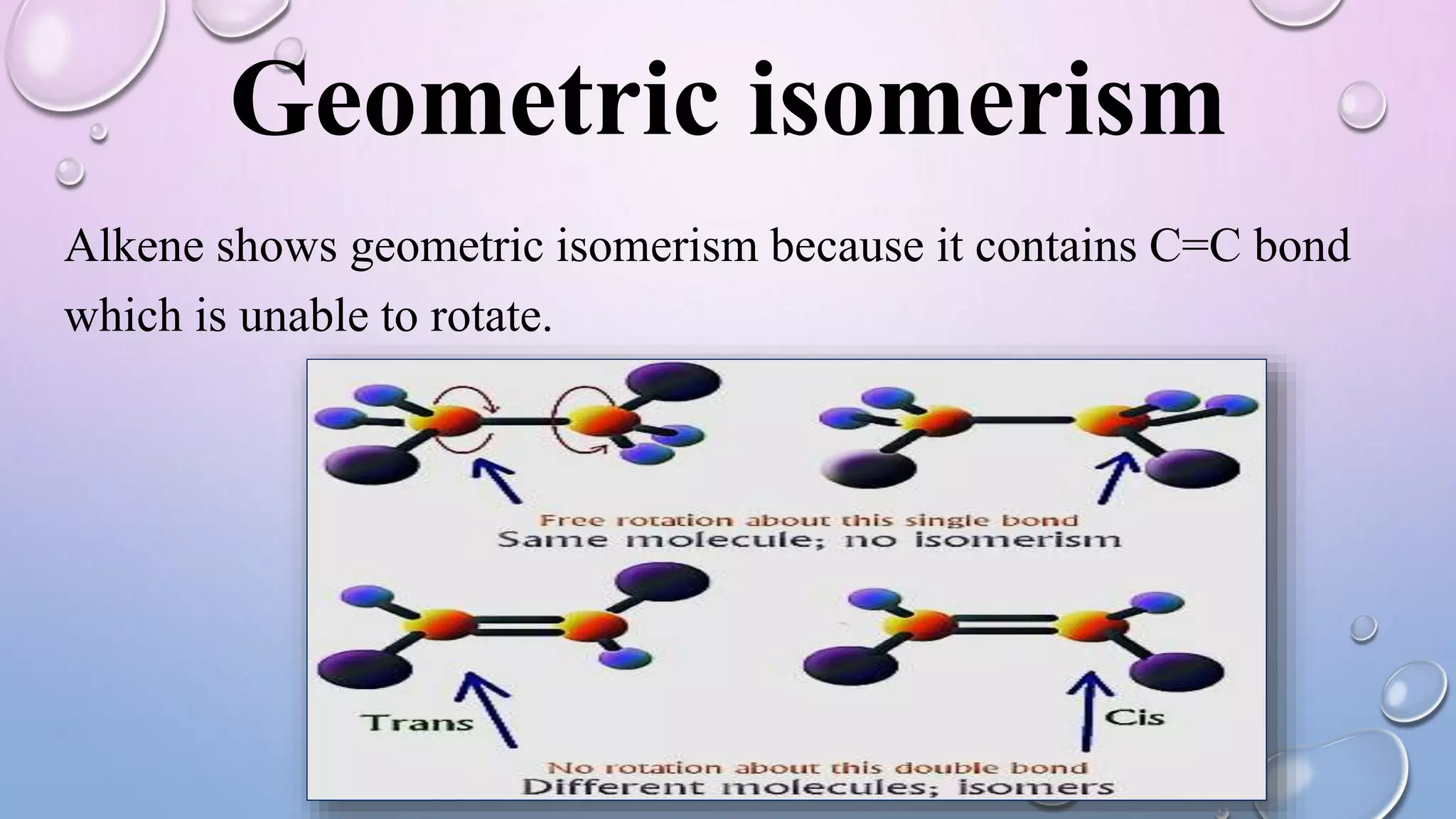
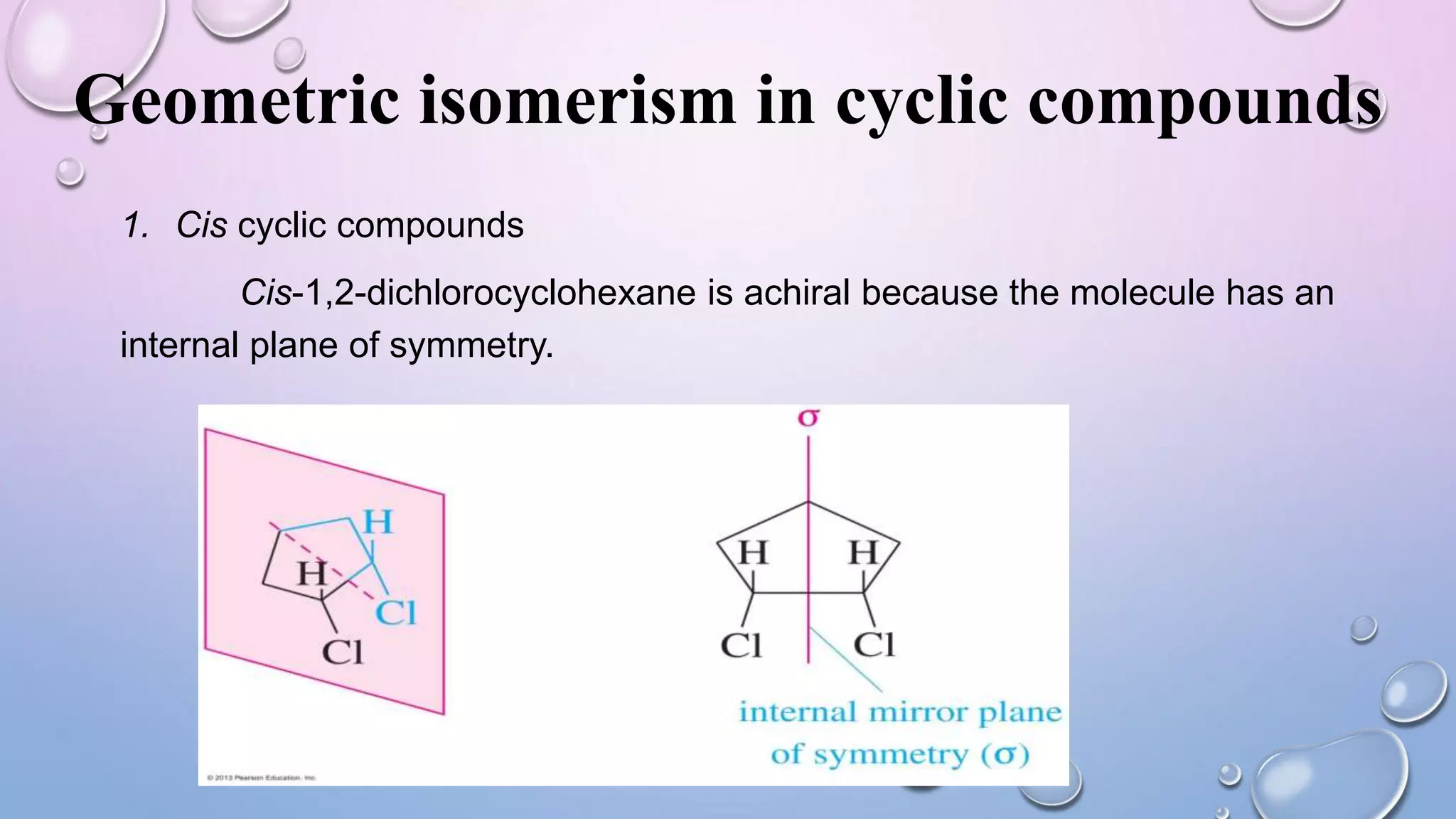
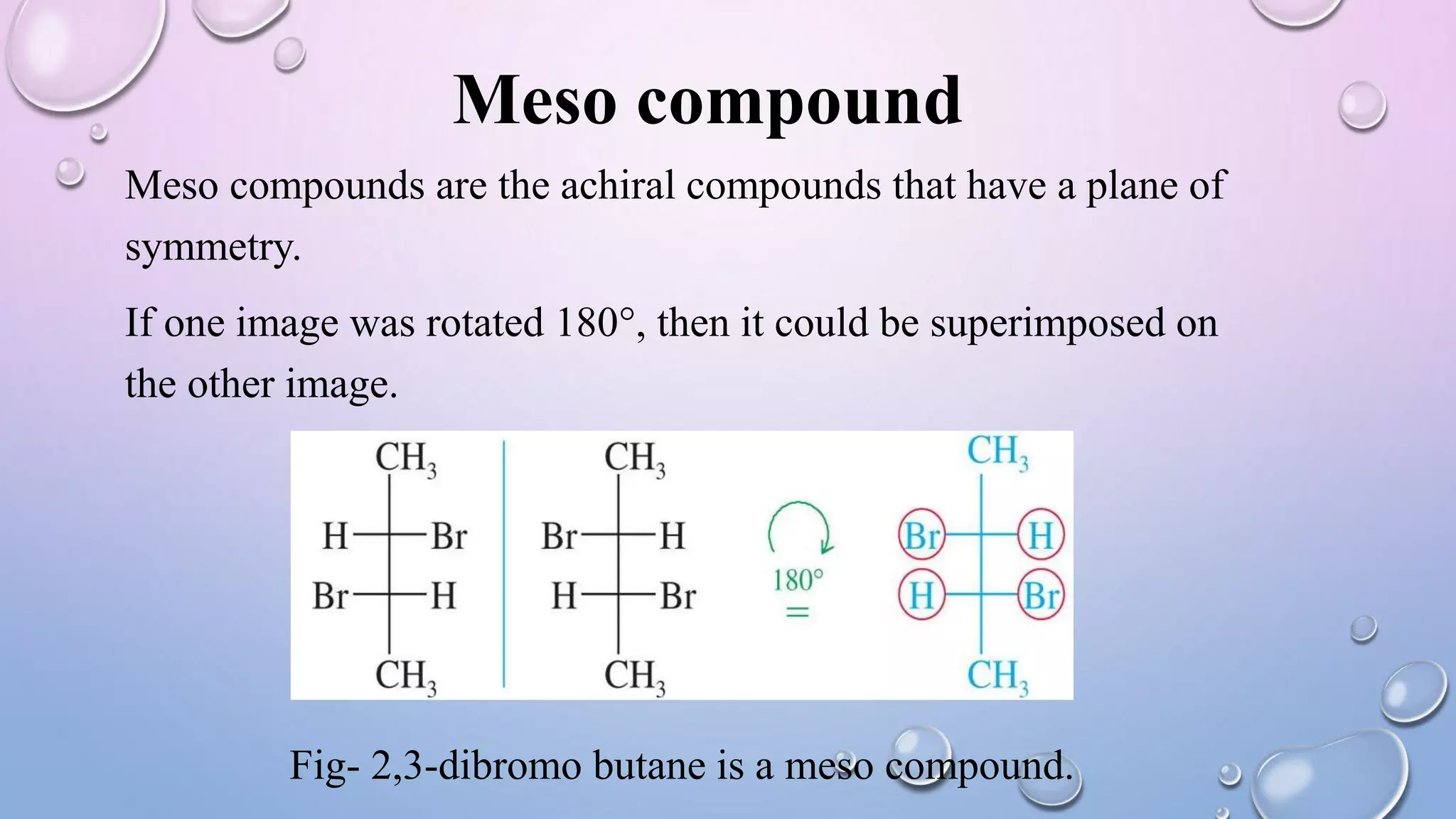
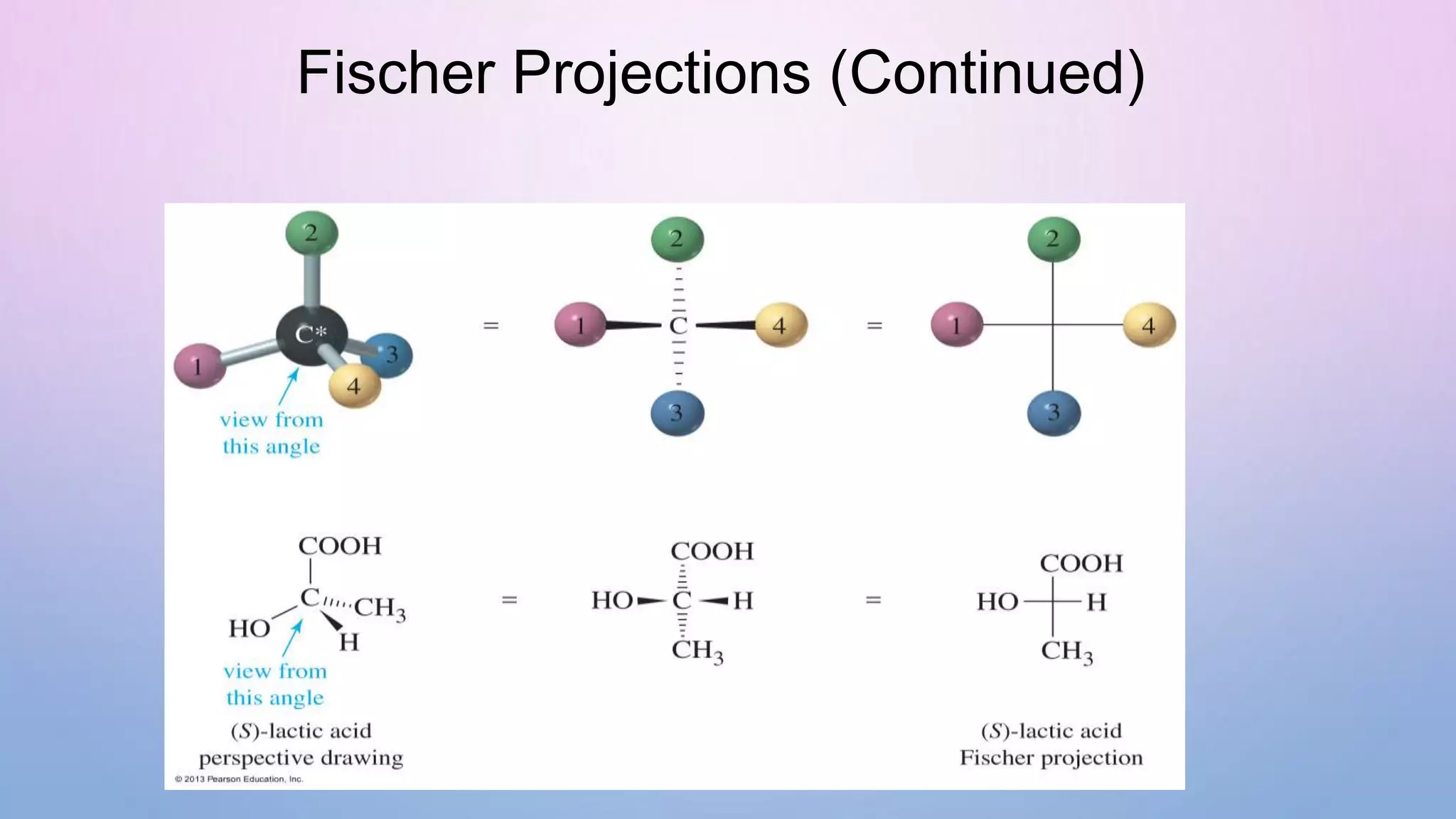
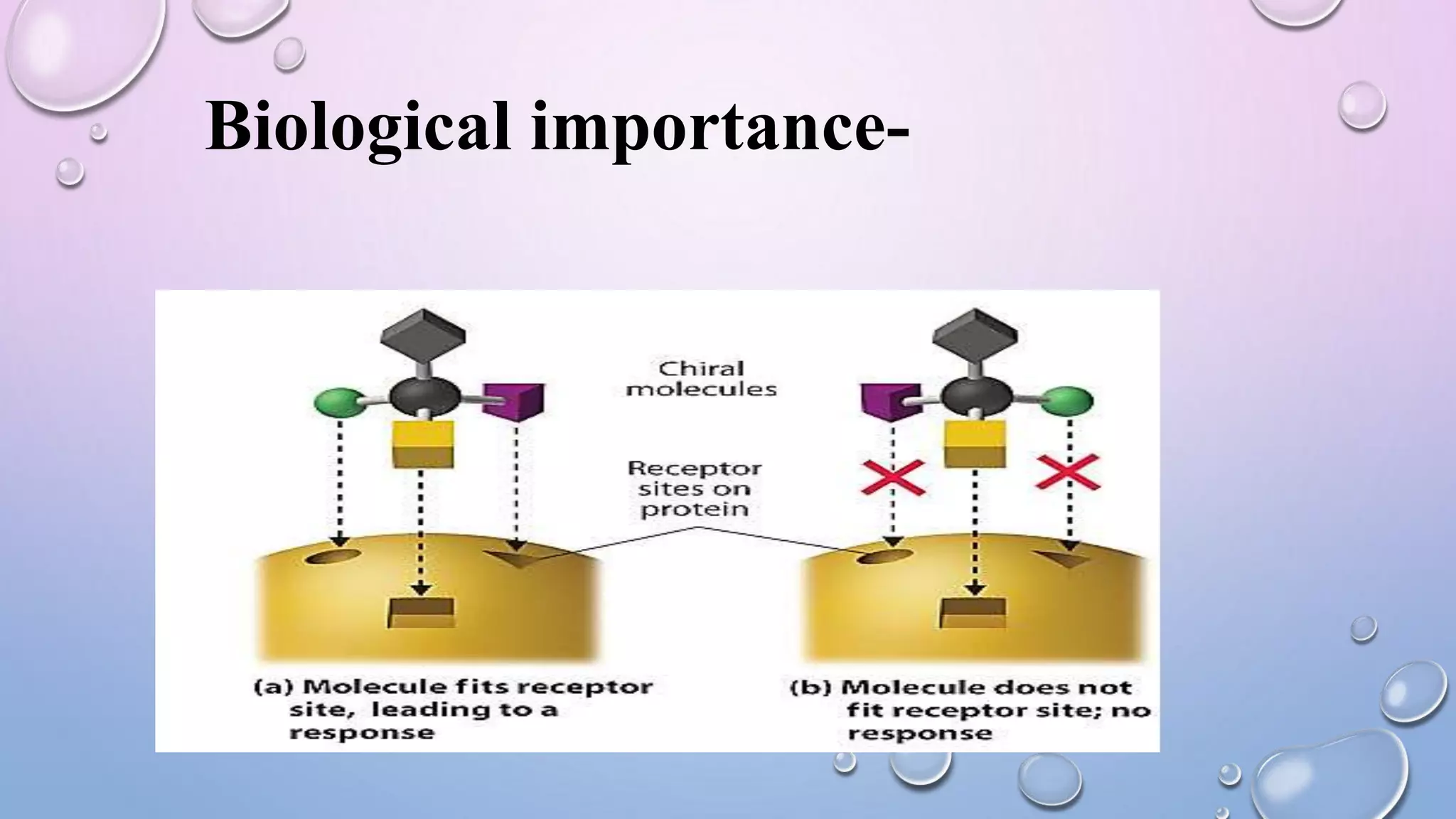
This document provides an outline and overview of stereochemistry concepts including isomerism, chiral and achiral carbons, enantiomers, diastereomers, geometric isomerism, asymmetric synthesis, and optical resolution methods. Key topics covered are classification of isomers, properties of chiral carbons, differences between enantiomers and diastereomers, cis-trans isomers in cyclic compounds, how asymmetric synthesis produces unequal product amounts, and methods for separating enantiomers including mechanical, biochemical and chemical separation. Applications of stereochemistry in medicinal compounds and biological systems are also briefly discussed.