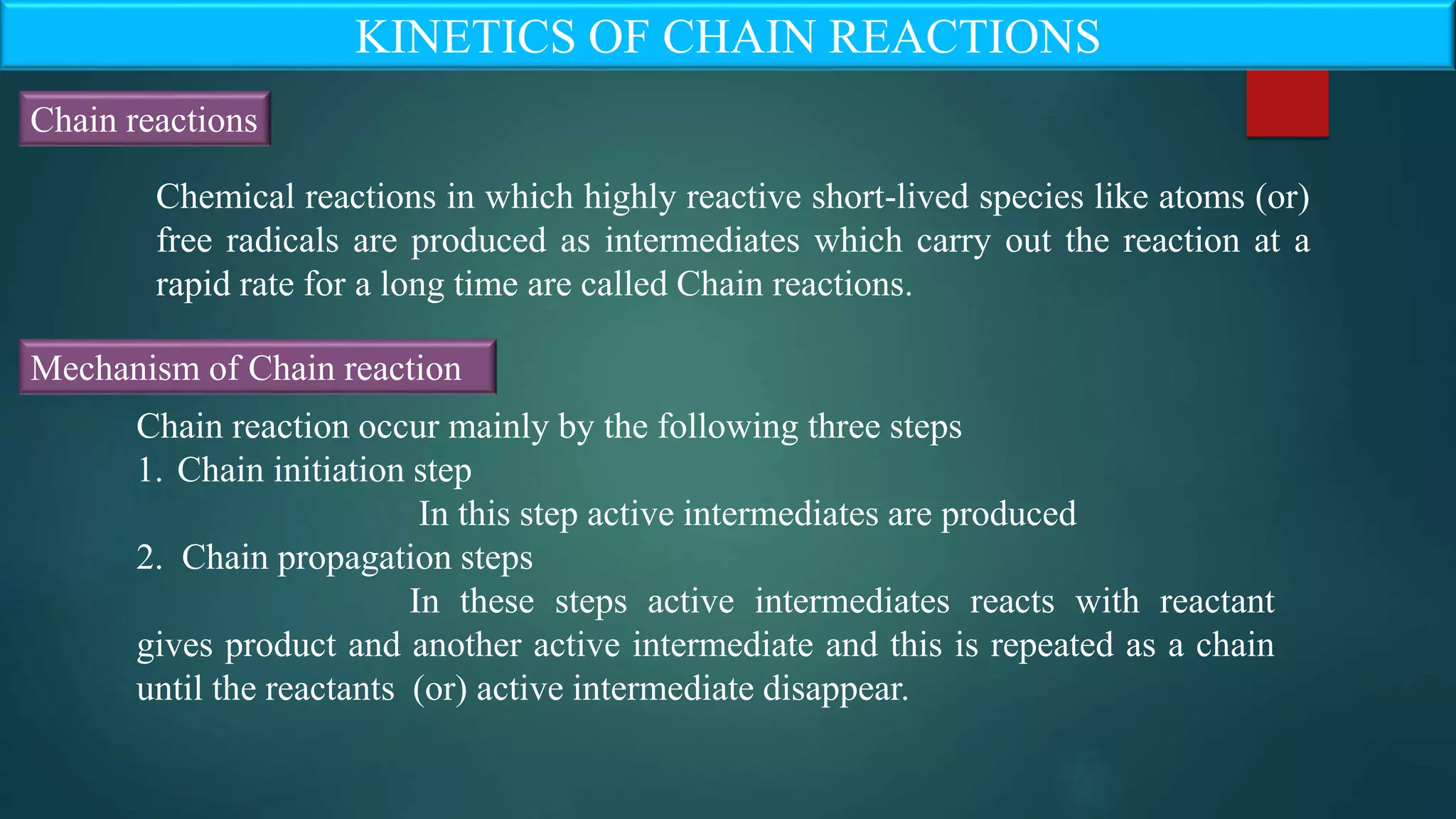
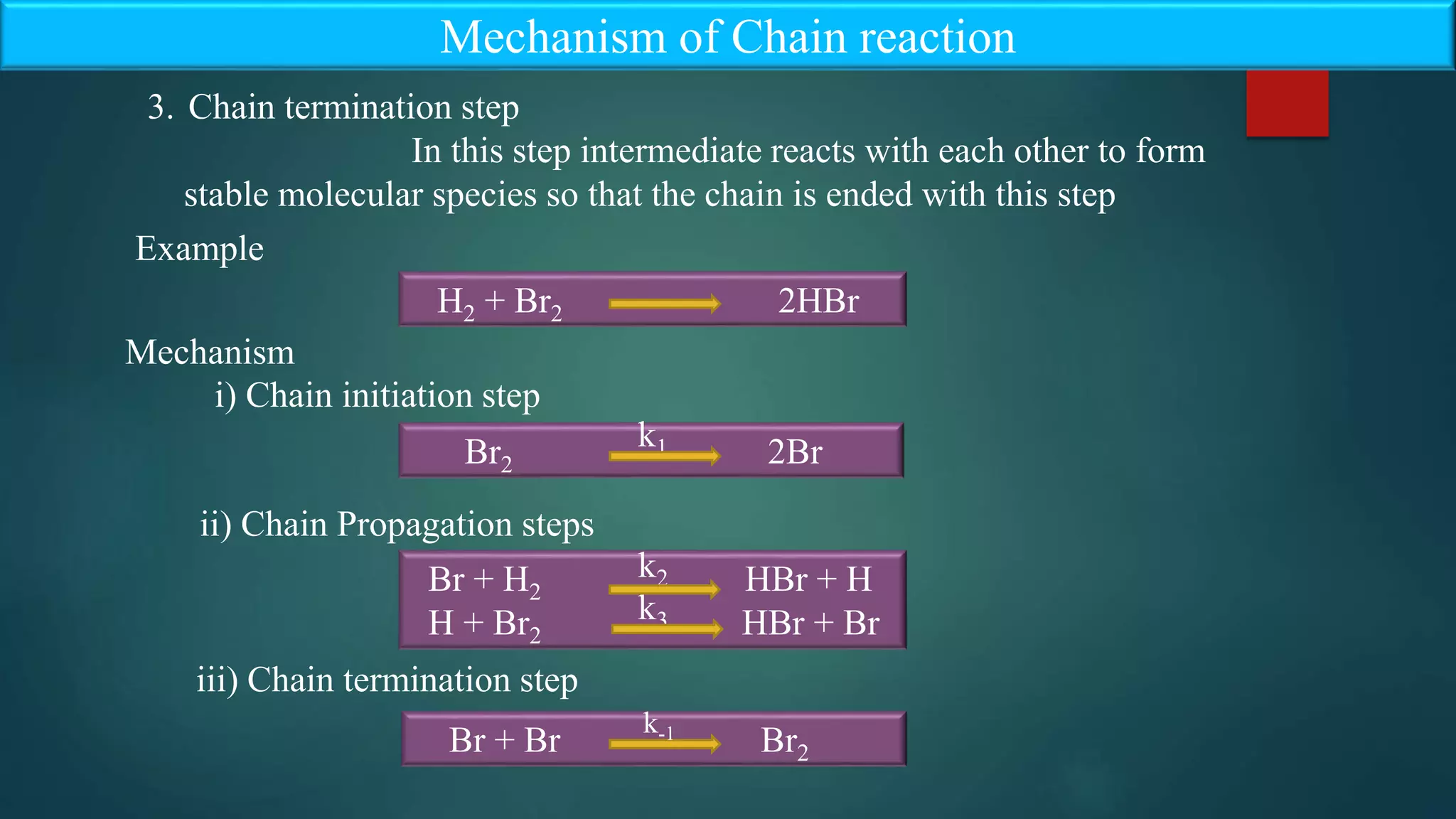
The document discusses the kinetics of chain reactions, highlighting their three main steps: initiation, propagation, and termination, with examples provided such as the reaction between hydrogen and bromine. It also outlines characteristics of chain reactions, like the probability factor often being greater than unity and the influence of external factors on reaction speed. The steady state approximation is explored in detail, demonstrating its application in the kinetics of thermal decomposition of acetaldehyde.


![vi) The rate of chain reactions is influenced by the changes in shape of the containing vessel.
vii)The chain reactions are rarely of simple orders but have integral orders which depends
upon the vessel shape and other experimental conditions.
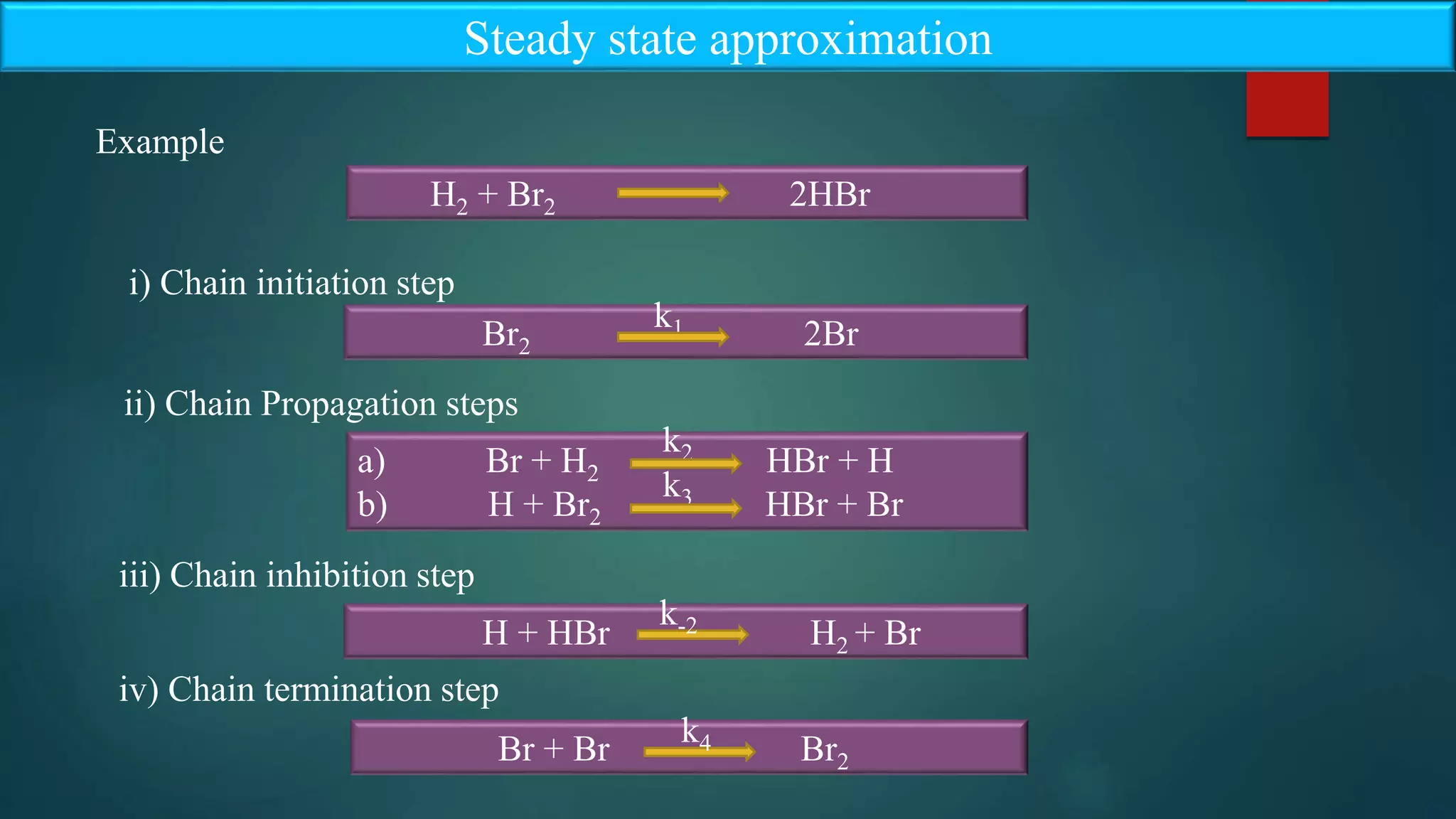
Steady state approximation states that the rate of formation of active intermediate is equal
to the rate of disappearance of active intermediate in the chain reactions
Steady state approximation
i.e., the rate of formation of intermediate (R) and the rate of disappearance of intermediate
(R) must be equal to zero
𝑑[𝑅]
𝑑𝑡
= −
𝑑[𝑅]
𝑑𝑡
= 0
Characteristics of chain reactions](https://image.slidesharecdn.com/kinetics-200423075918/75/Kinetics-of-Chain-reaction-5-2048.jpg)
![On applying steady state approximation to the intermediate H
𝑑[𝐻]
𝑑𝑡
= Rate of formation of [H] – Rate of disappearance of [H] = 0 ------(1)
Rate of formation of [H] = k2[Br][H2] ------(2)
Rate of disappearance of [H] = k3[H][Br2] + k-2[H][HBr] ------(3)
Substitute (2) and (3) in (1) we get
𝑑[𝐻]
𝑑𝑡
= k2[Br][H2] - k3[H][Br2] - k-2[H][HBr] = 0
Steady state approximation](https://image.slidesharecdn.com/kinetics-200423075918/75/Kinetics-of-Chain-reaction-7-2048.jpg)

![i.e., Rate of formation of methyl radical = Rate of disappearance of methyl radical
𝑑[𝐶𝐻4
]
𝑑𝑡
= k2[CH3][CH3CHO] ------(1)
Applying steady state approximation on methyl radicals
The rate of formation of Methane is
k1[CH3CHO] + k3[CH3CO] = k2[CH3][CH3CHO] + k4[CH3][CH3]
k1[CH3CHO] + k3[CH3CO] - k2[CH3][CH3CHO] - k4[CH3][CH3] = 0 ------(2)
Kinetics of thermal decomposition of Acetaldehyde](https://image.slidesharecdn.com/kinetics-200423075918/75/Kinetics-of-Chain-reaction-9-2048.jpg)
![Applying steady state approximation on CH3CO radicals
i.e., Rate of formation of CH3CO = Rate of disappearance of CH3CO
k2[CH3][CH3CHO] = k3[CH3CO]
k2[CH3][CH3CHO] - k3[CH3CO]= 0 ------(3)
Adding equation (2) and (3) we get the concentration of methyl radicals
k1[CH3CHO] + k3[CH3CO] - k2[CH3][CH3CHO] - k4[CH3][CH3] + k2[CH3][CH3CHO] -
k3[CH3CO] = 0
k1[CH3CHO] - k4[CH3]2 = 0
k1[CH3CHO] = k4[CH3]2
Kinetics of thermal decomposition of Acetaldehyde](https://image.slidesharecdn.com/kinetics-200423075918/75/Kinetics-of-Chain-reaction-10-2048.jpg)
![k1[CH3CHO] = k4[CH3]2
[CH3]2 =
k1
k4
[CH3CHO]
k4[CH3]2 = k1[CH3CHO]
[CH3] = (
k1
k4
)1/2[CH3CHO]1/2
-------(4)
Substitute (4) in (1) we get
𝑑[𝐶𝐻4]
𝑑𝑡
= k2(
k1
k4
)1/2[CH3CHO]1/2 [CH3CHO]
𝑑[𝐶𝐻4]
𝑑𝑡
= k2(
k1
k4
)1/2[CH3CHO]3/2
This is the rate equation for thermal decomposition of CH3CHO and the
overall order of this reaction is 3/2
Kinetics of thermal decomposition of Acetaldehyde](https://image.slidesharecdn.com/kinetics-200423075918/75/Kinetics-of-Chain-reaction-11-2048.jpg)
