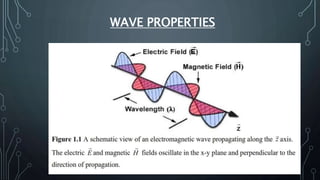
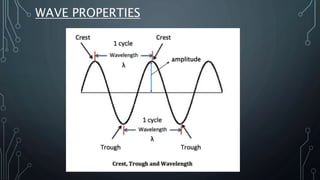
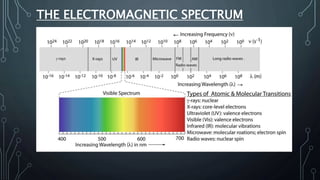
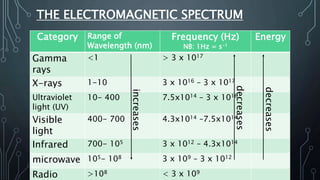
This document discusses electromagnetic radiation and its properties. It defines electromagnetic radiation as a form of energy that exhibits both wave and particle properties. As a wave, electromagnetic radiation is characterized by its frequency, wavelength, and amplitude. Higher frequency radiation like gamma rays and X-rays have shorter wavelengths and higher energies, allowing them to penetrate deeper into materials. Exposure to high energy radiation can be dangerous by breaking molecular bonds through ionization. Electromagnetic radiation can also be described by photon particles, where the energy of each photon is determined by Planck's constant and the radiation's frequency or wavelength.