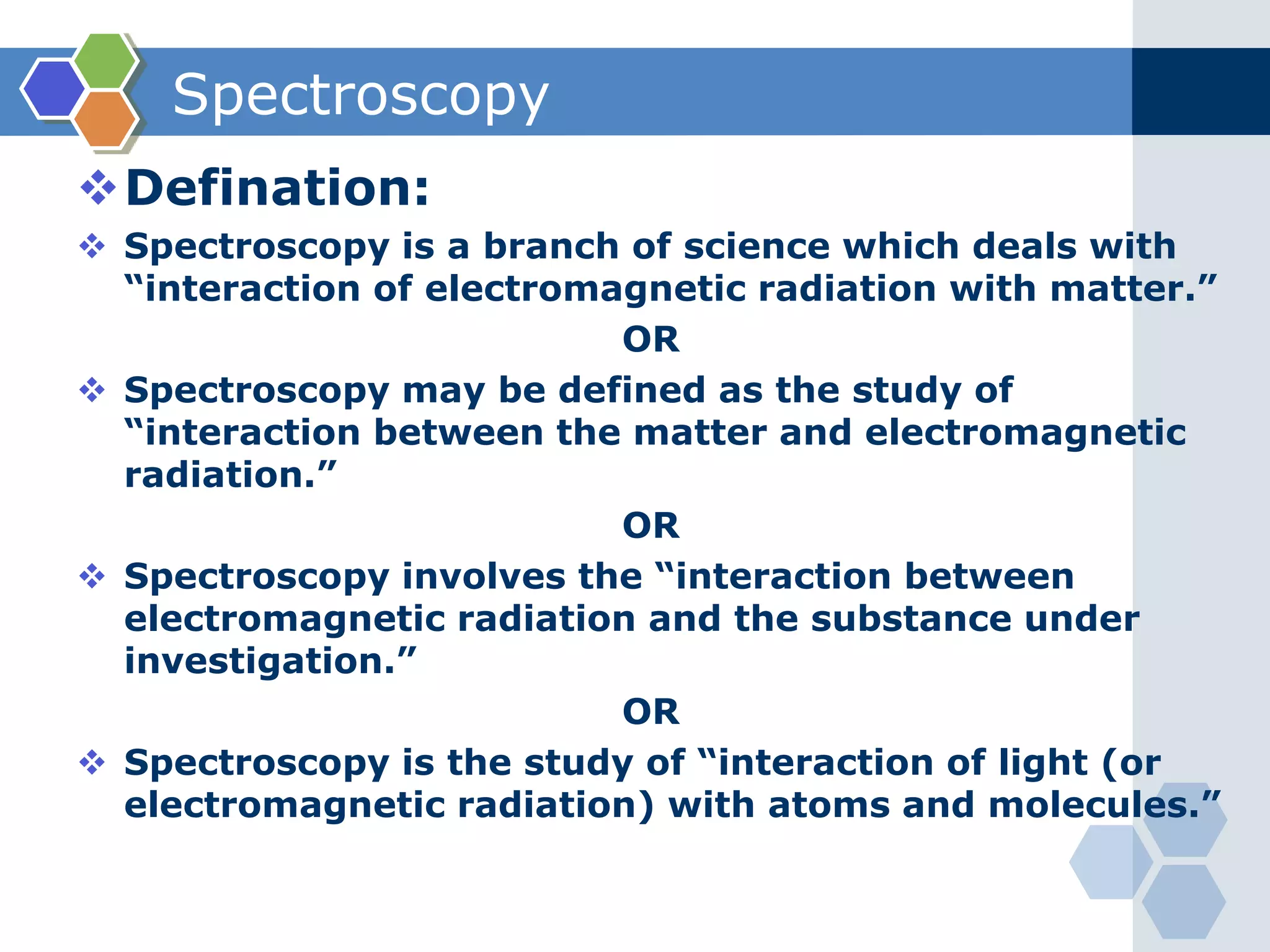
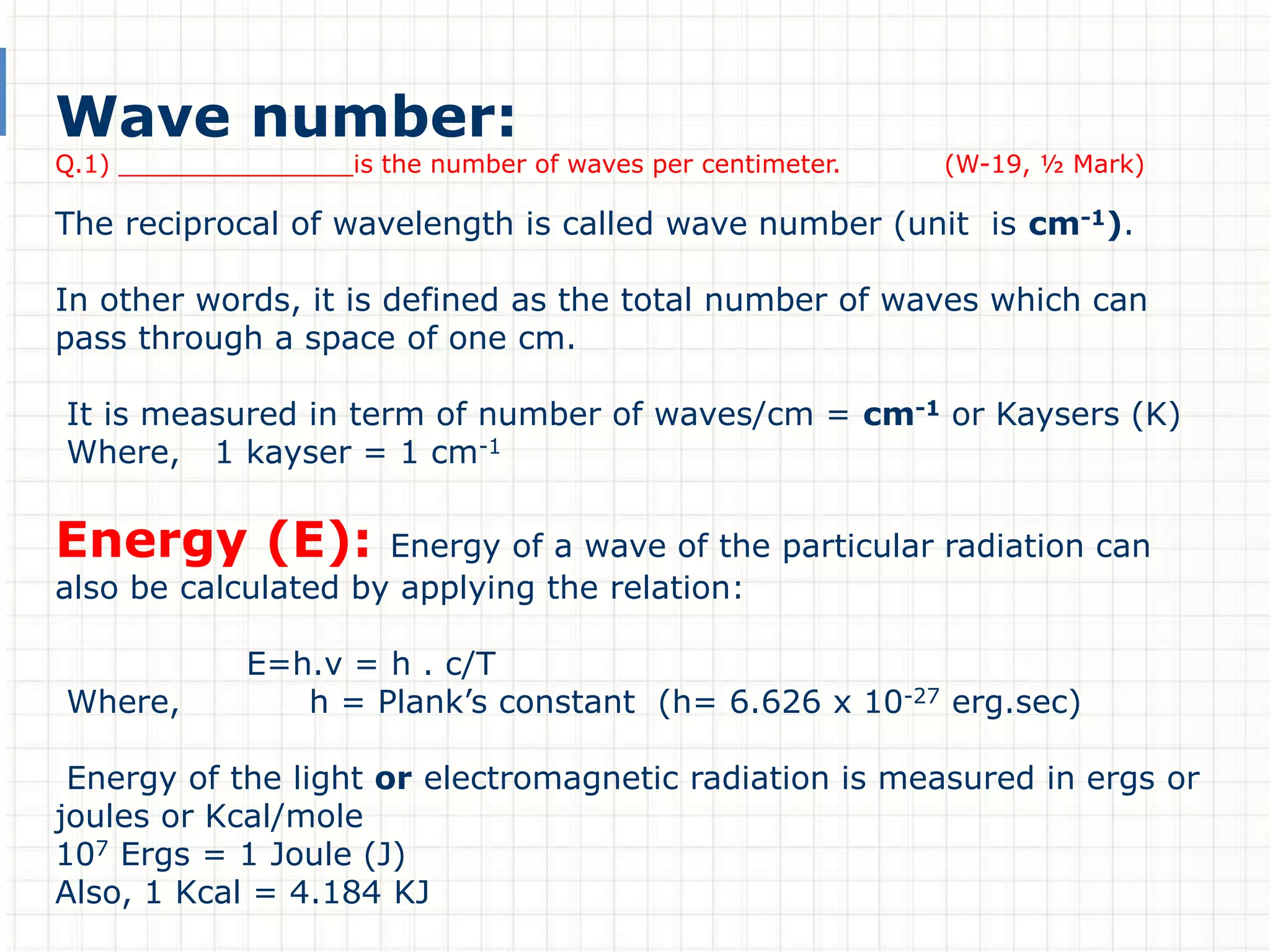
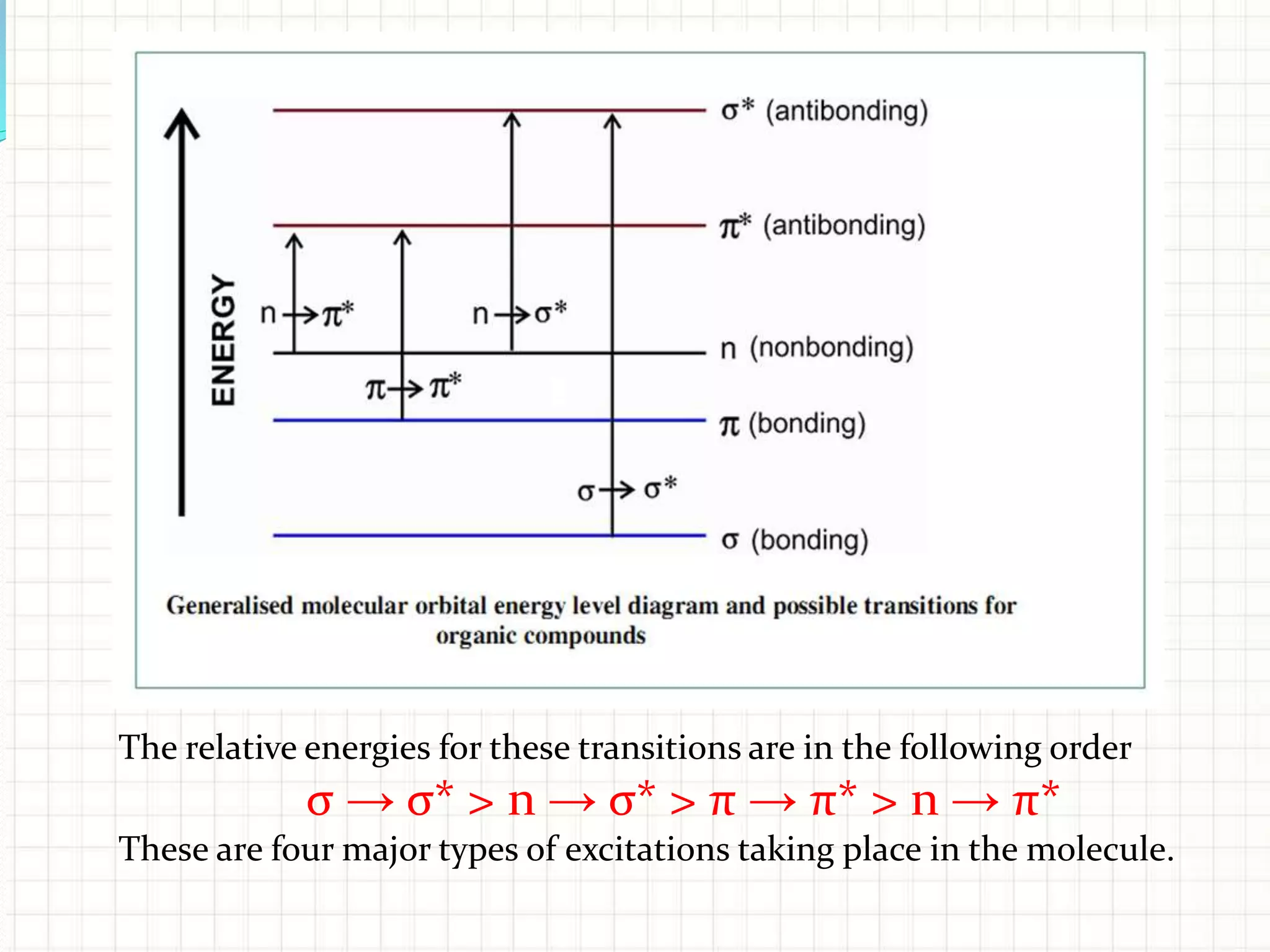
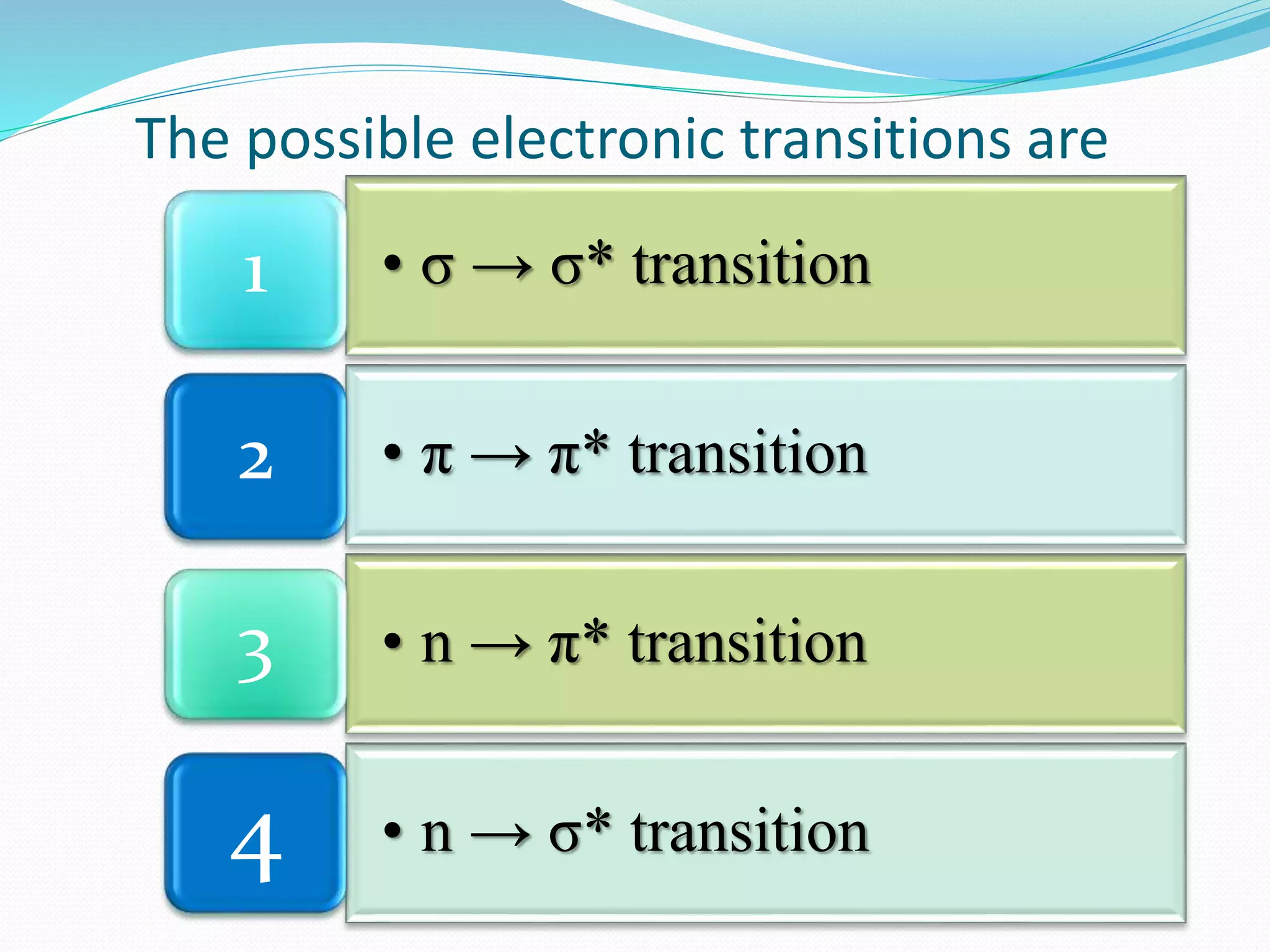
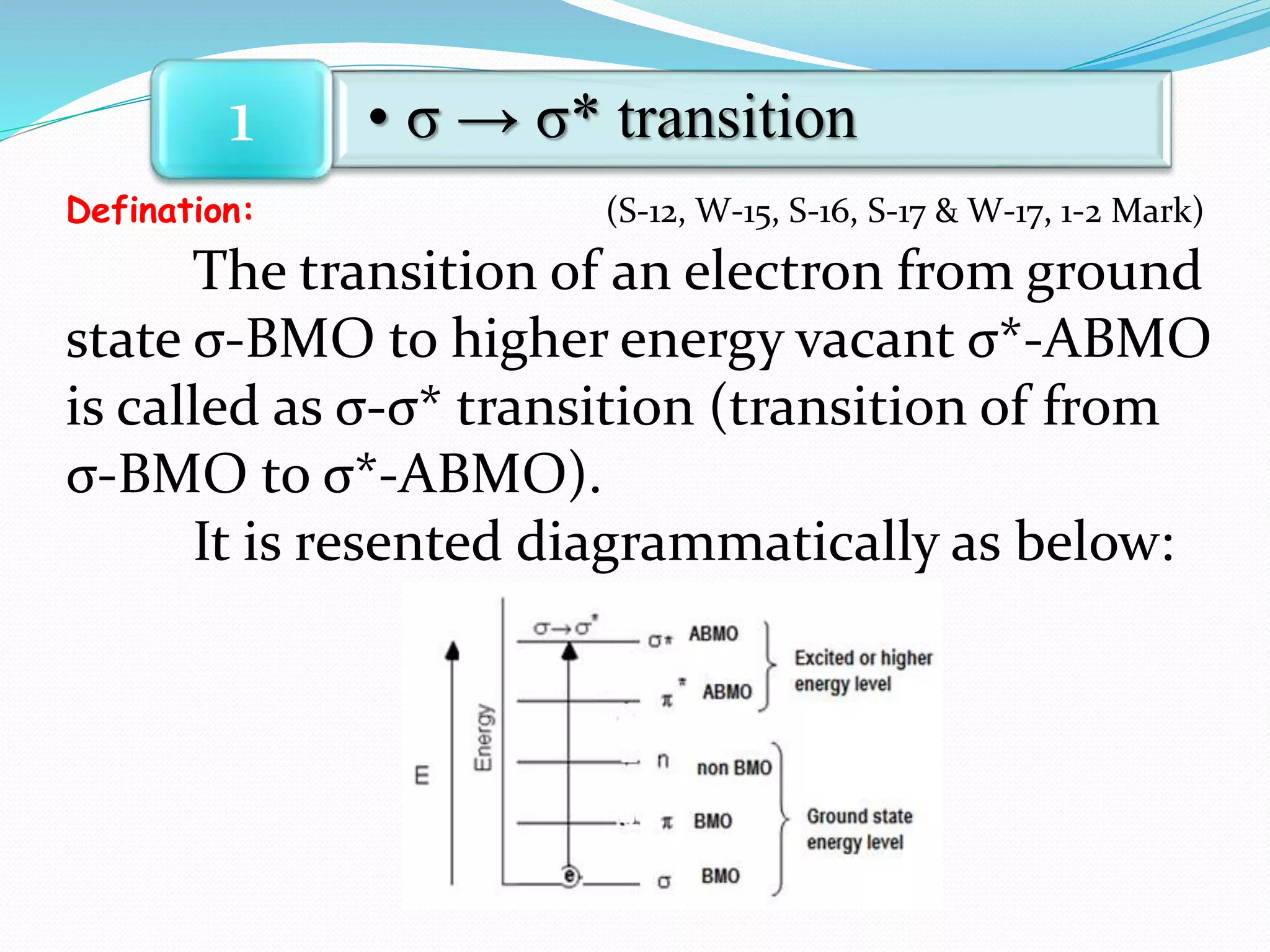
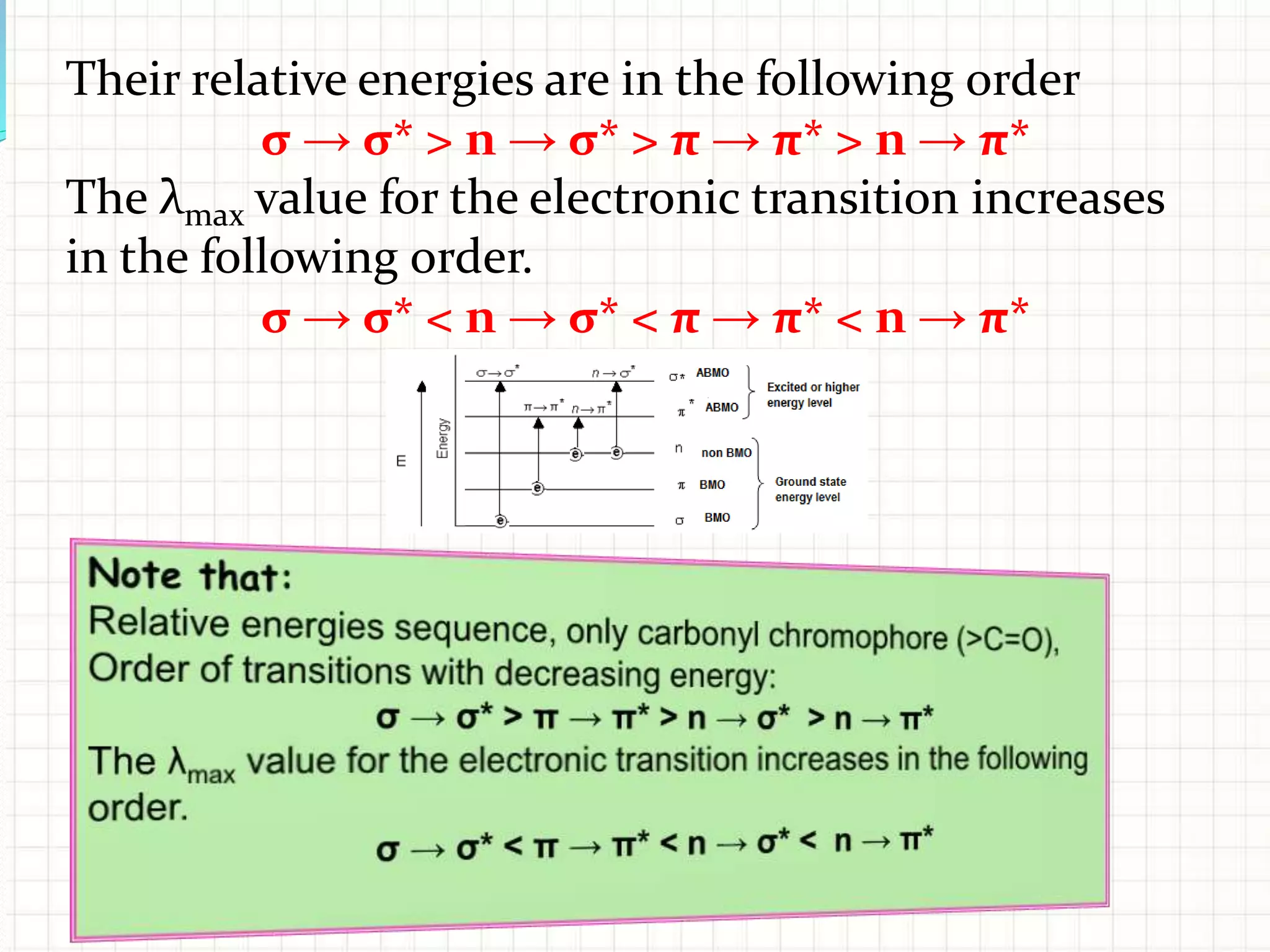
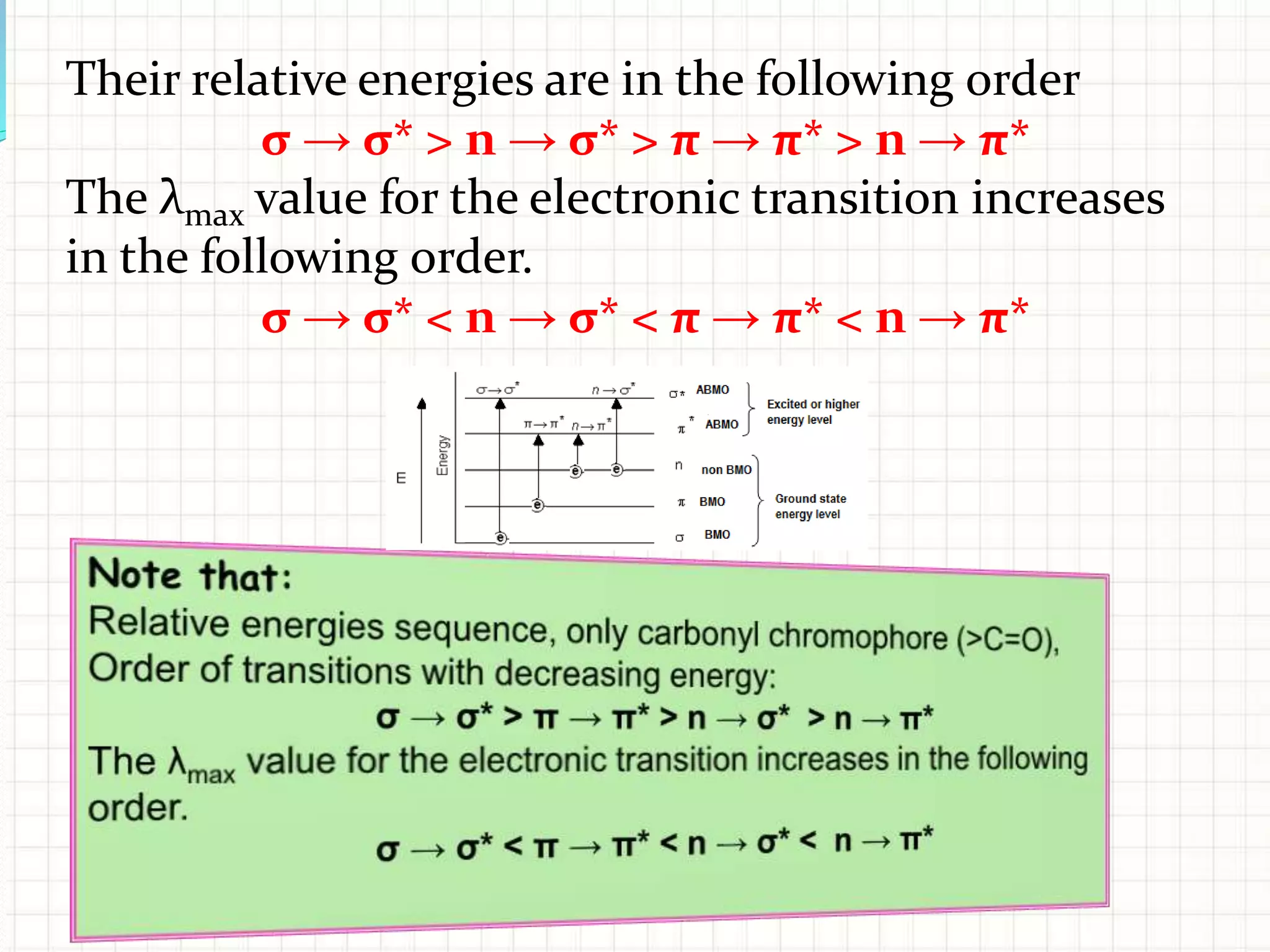
There are three main types of electronic transitions that can occur in organic molecules:
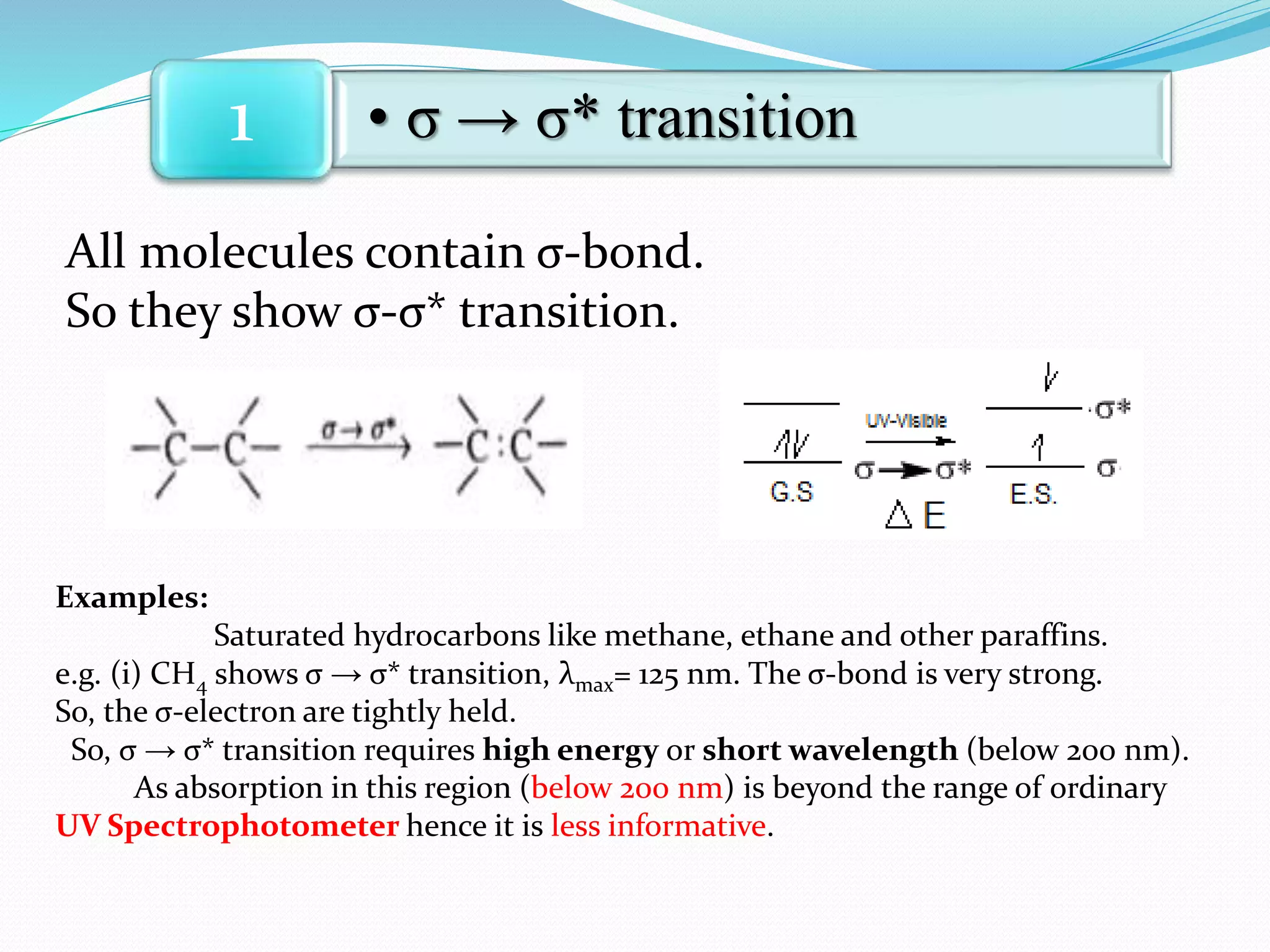
1. σ → σ* transitions: These involve promotion of electrons from bonding σ orbitals to antibonding σ* orbitals. They require high energy in the far UV region (below 200 nm) and are usually not observed for organic compounds.
2. n → π* transitions: These involve promotion of electrons from non-bonding n orbitals to antibonding π* orbitals. They occur in the near UV region (200-400 nm). Examples include carbonyl (C=O) and nitro (NO2) groups.
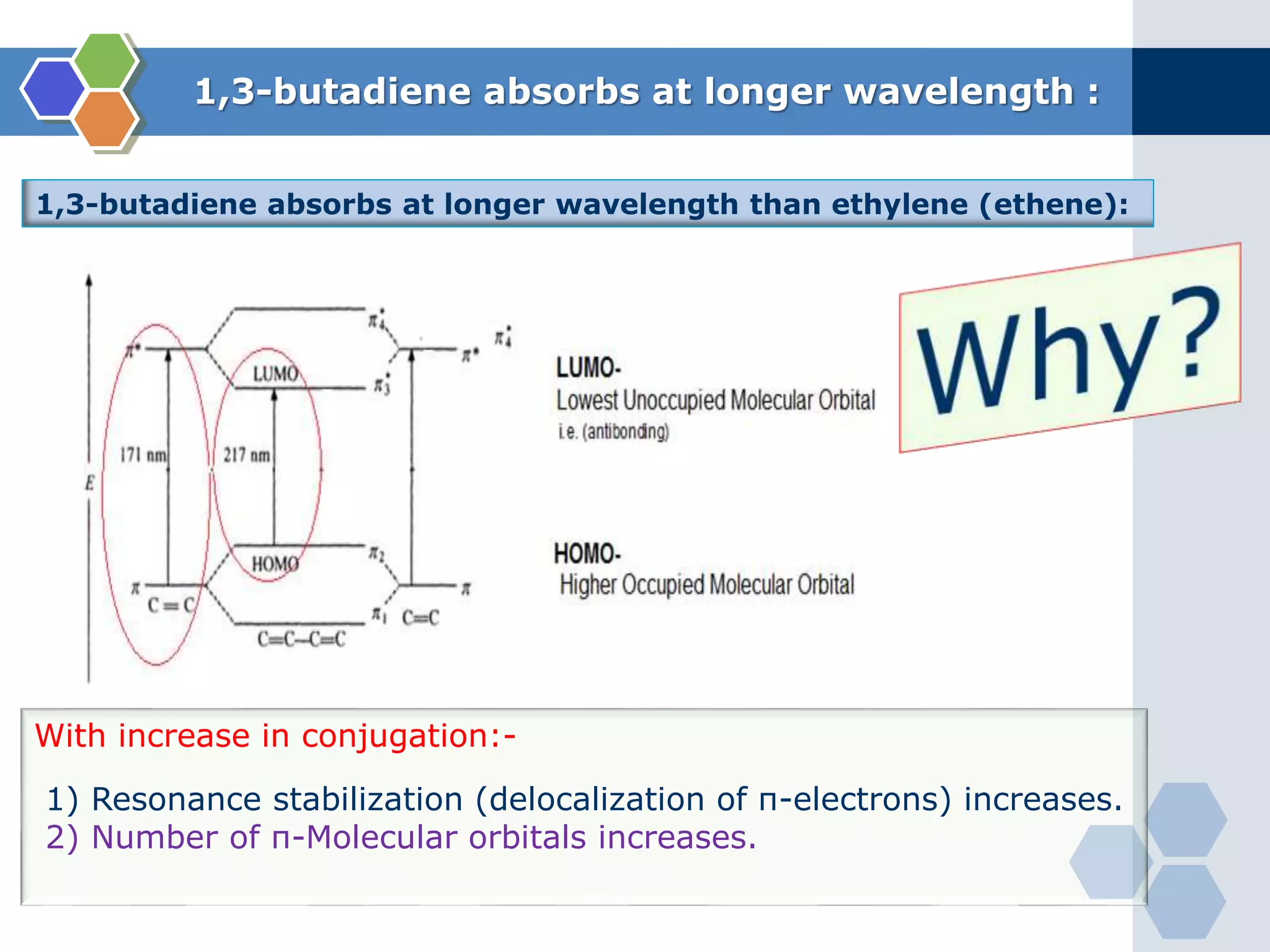
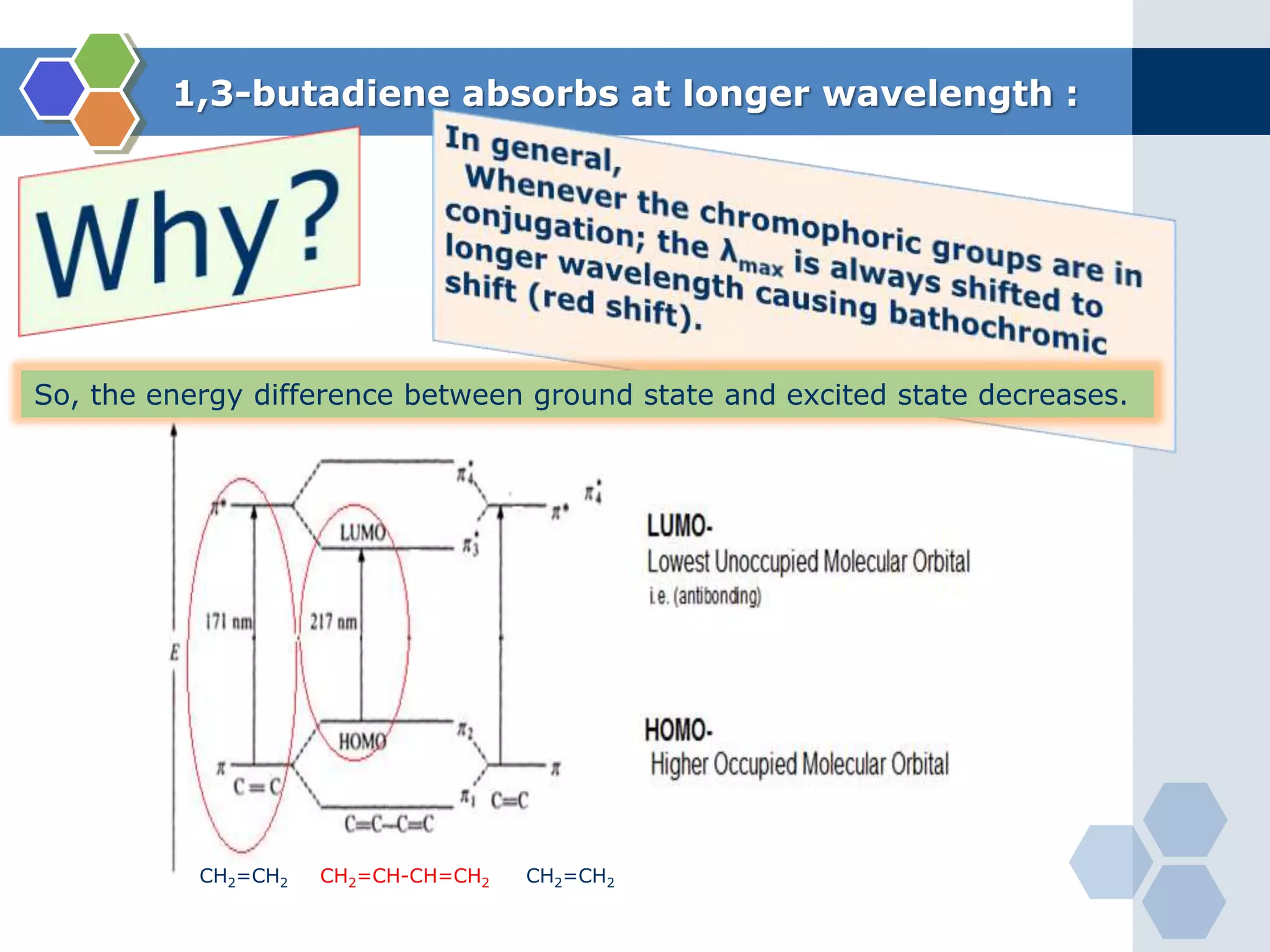
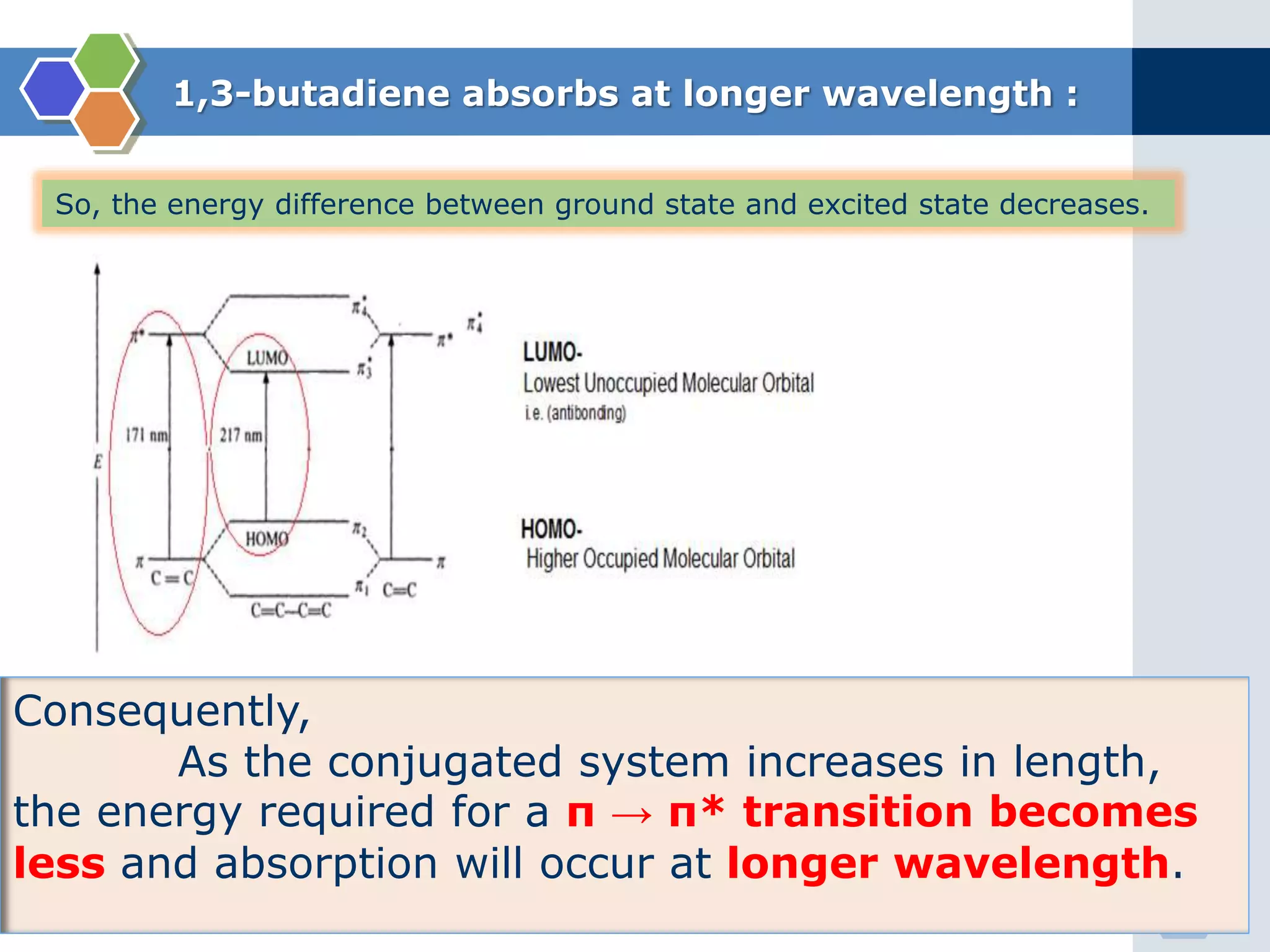
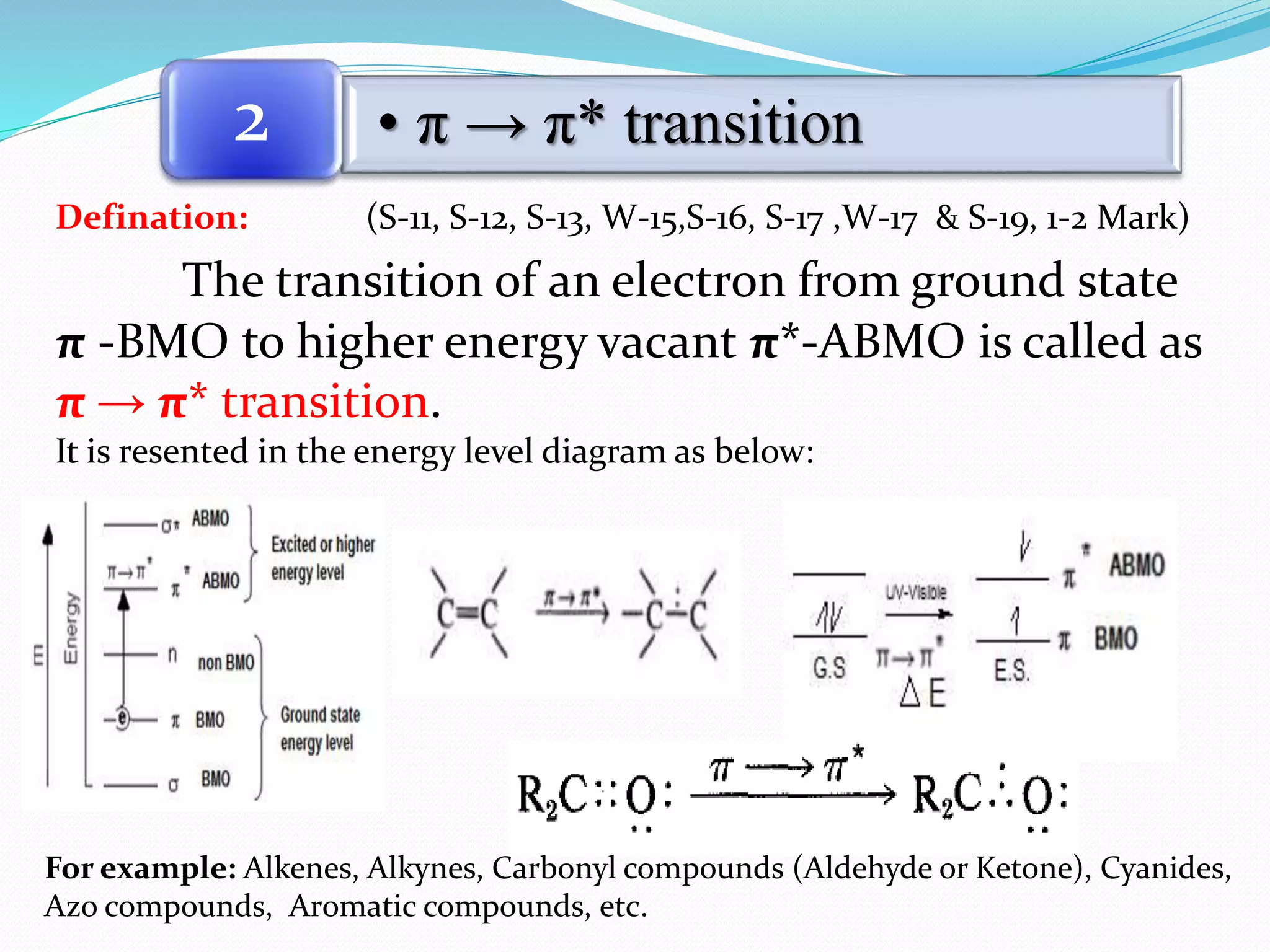
3. π → π* transitions: These involve promotion of electrons from bonding





















































![Chromophore:
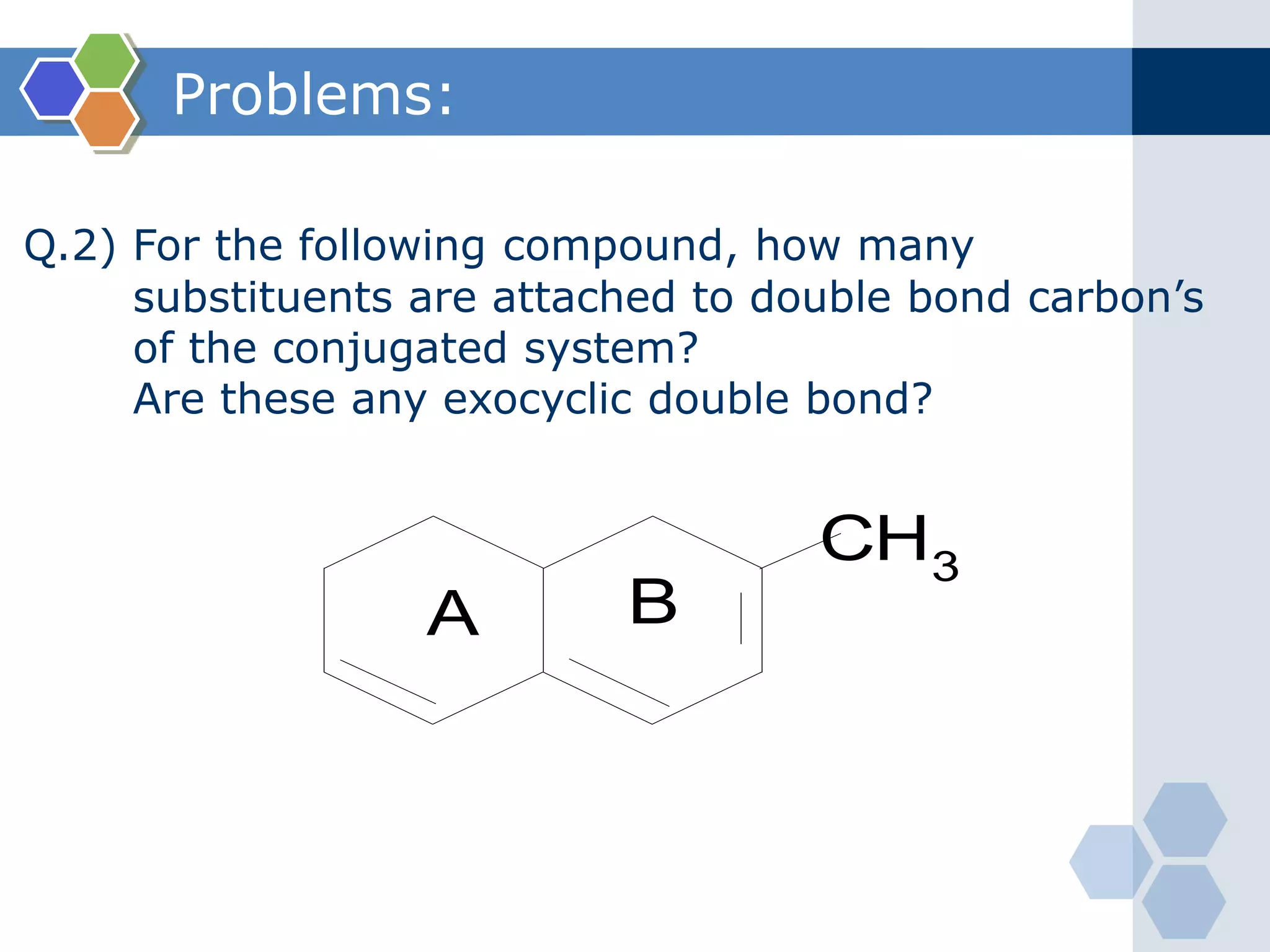
Q.1) Define and explains: (i) Chromophore with suitable examples.
(W-09, S-10, W-11, W-13, S-14, W-14, S-16, W-16, S-17, W-18 & W-19, 1-2 Mark)
Chromophore: [Greek: Chromo= colour, Phores= bearer]
Defination:
The unsaturated group which gives colour to the
compound (substance) by absorbing UV-Visible
radiation (light) is called as chromophore.
It contains π-electron.
Examples: Some important chromophores are ;
>C=C<, -CΞC-, ─N═O (nitroso), ─NO2,
─N═N- (azo), >C═O, >C═S (Thio),
─C≡N, etc.
The compounds bearing chromophores are known as chromogens.](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-89-2048.jpg)

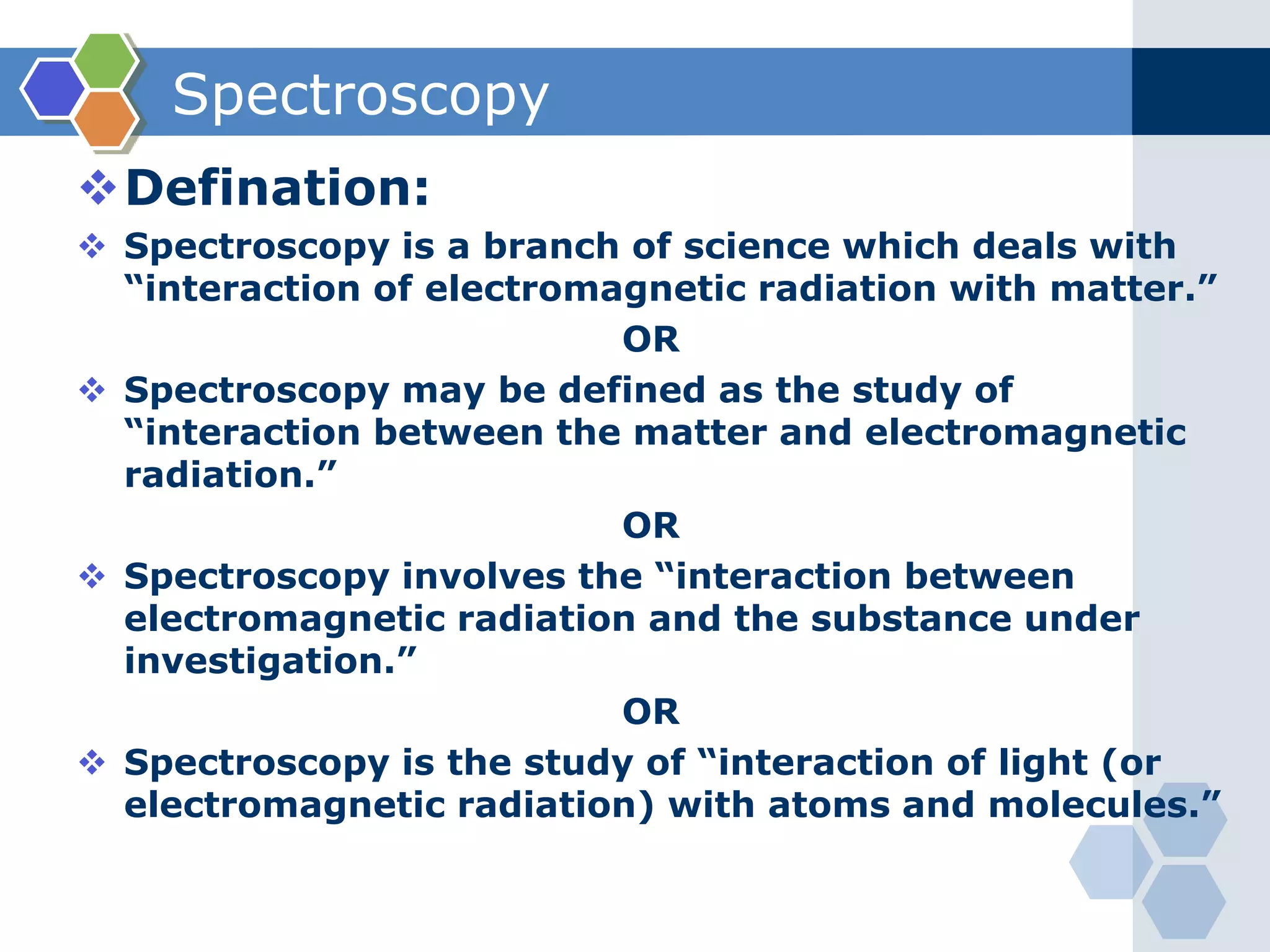
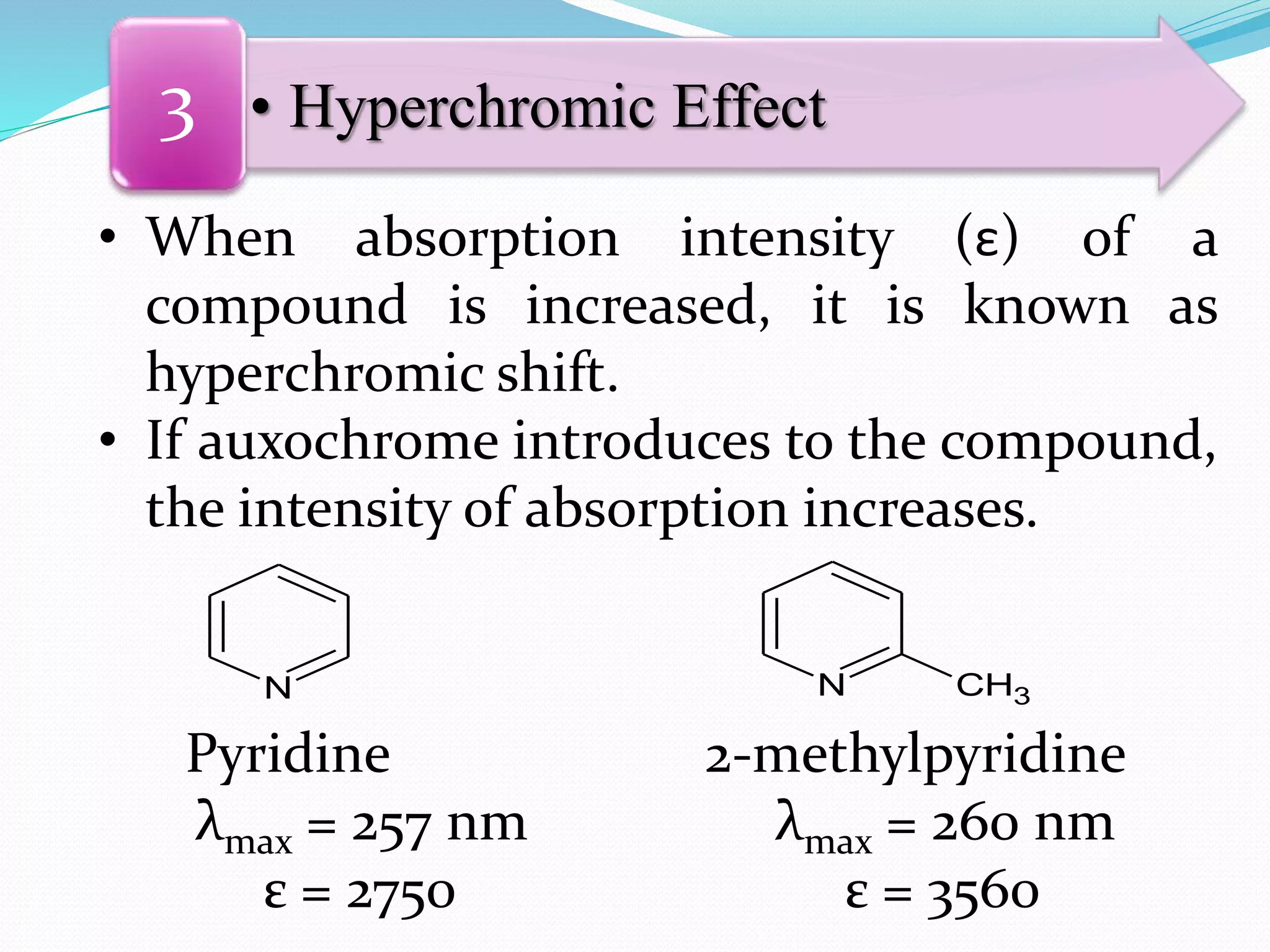
![Auxochrome:
Q.1) Define & explain the term: Auxochrome with suitable examples.
(W-09, S-10, W-13, W-14, S-16, W-16, W-17 & S-19, 1-2 Mark)
Auxochrome:
(increases intensity of colour & λmax of chromophore)
[Greek: Auxanein= to increase; Chroma= colour]
Defination:
The saturated group containing lone pair of
electrons which increases intensity of colour and
wavelength of maximum absorption (λmax) of
chromophore is called as auxochrome.
It contains non-bonding electron (lone-pair electrons).
e.g. -OH, -NH2, -X (X=Cl,Br, I), etc. are
auxochrome.](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-91-2048.jpg)






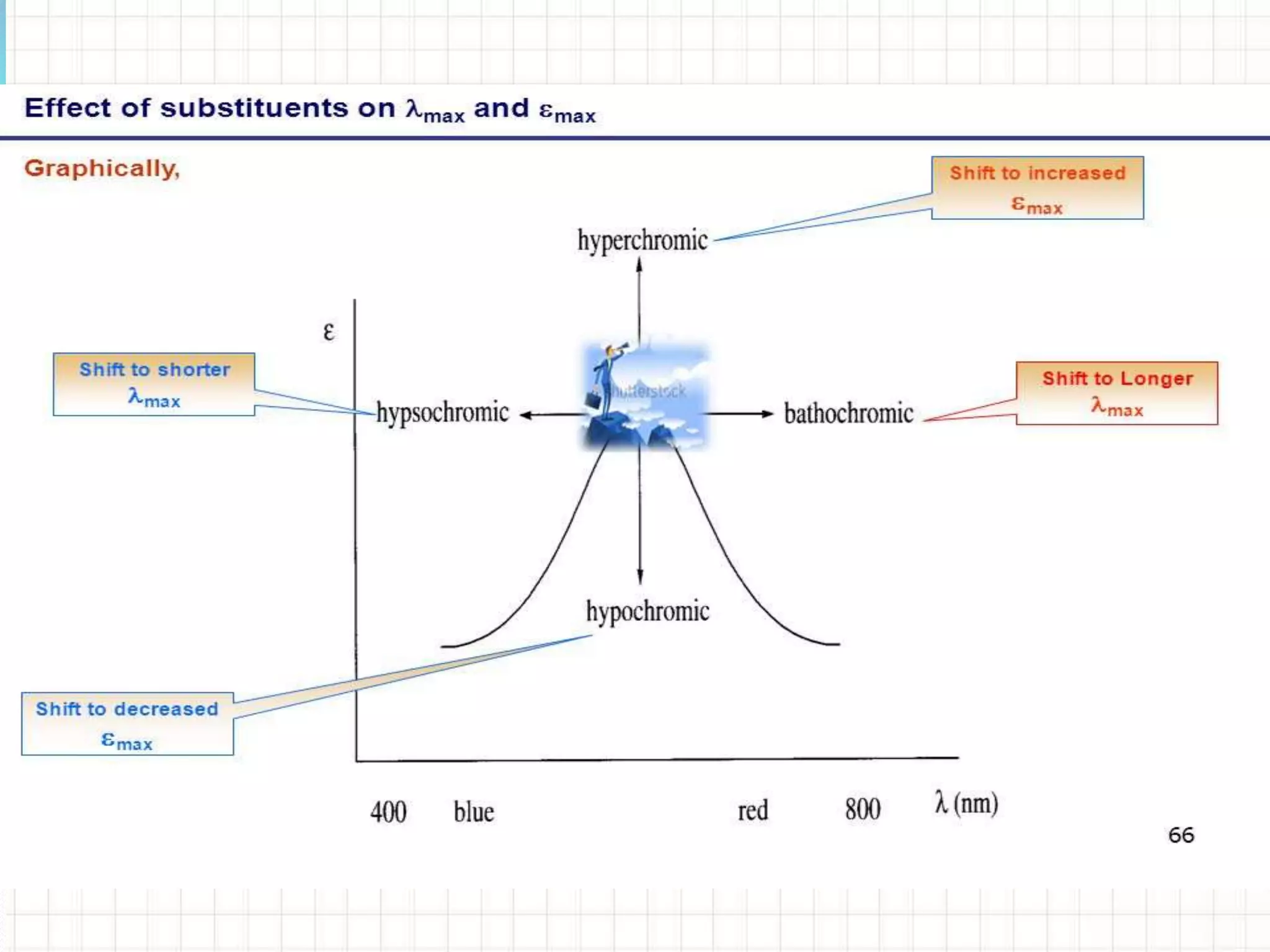
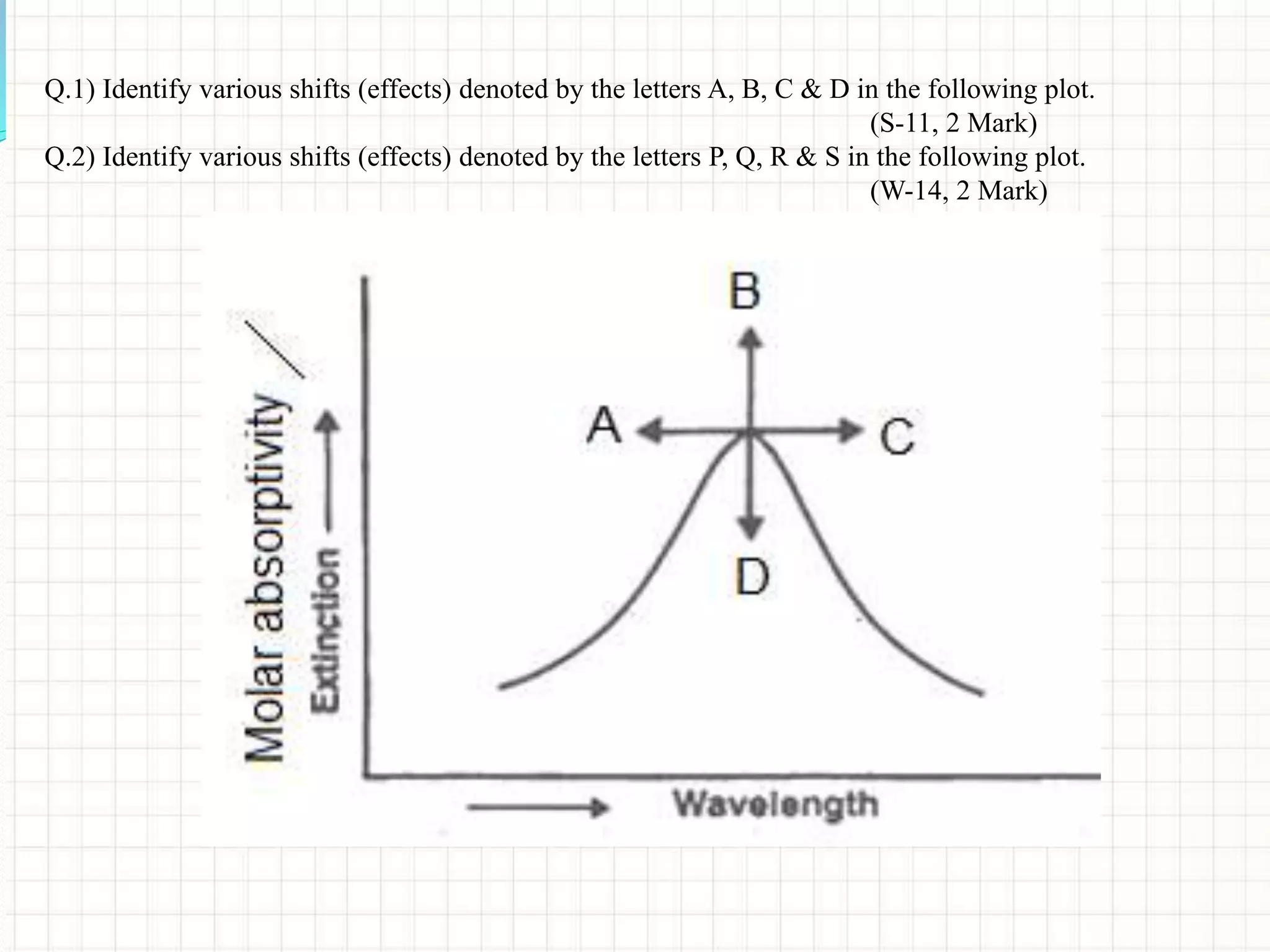

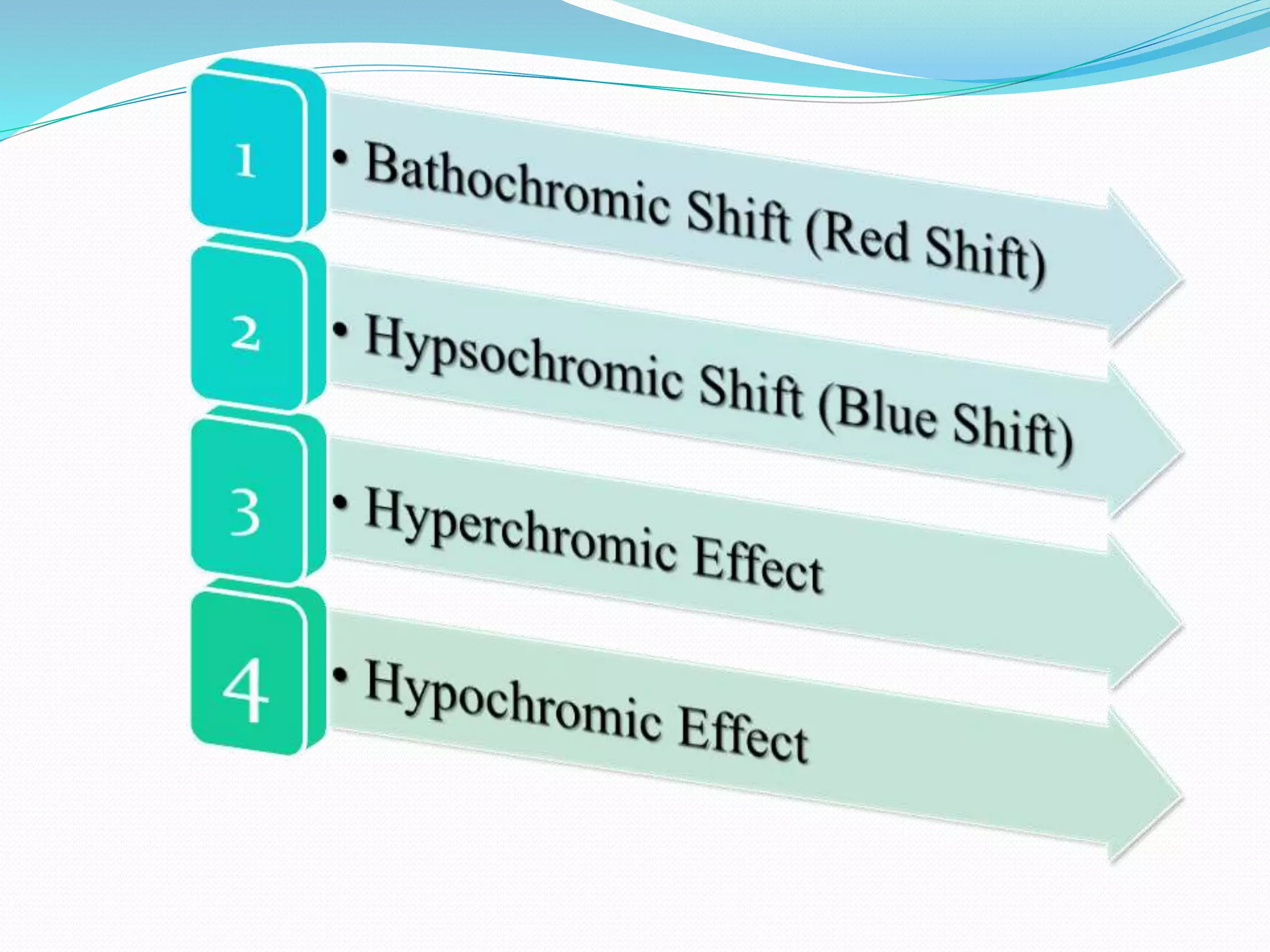


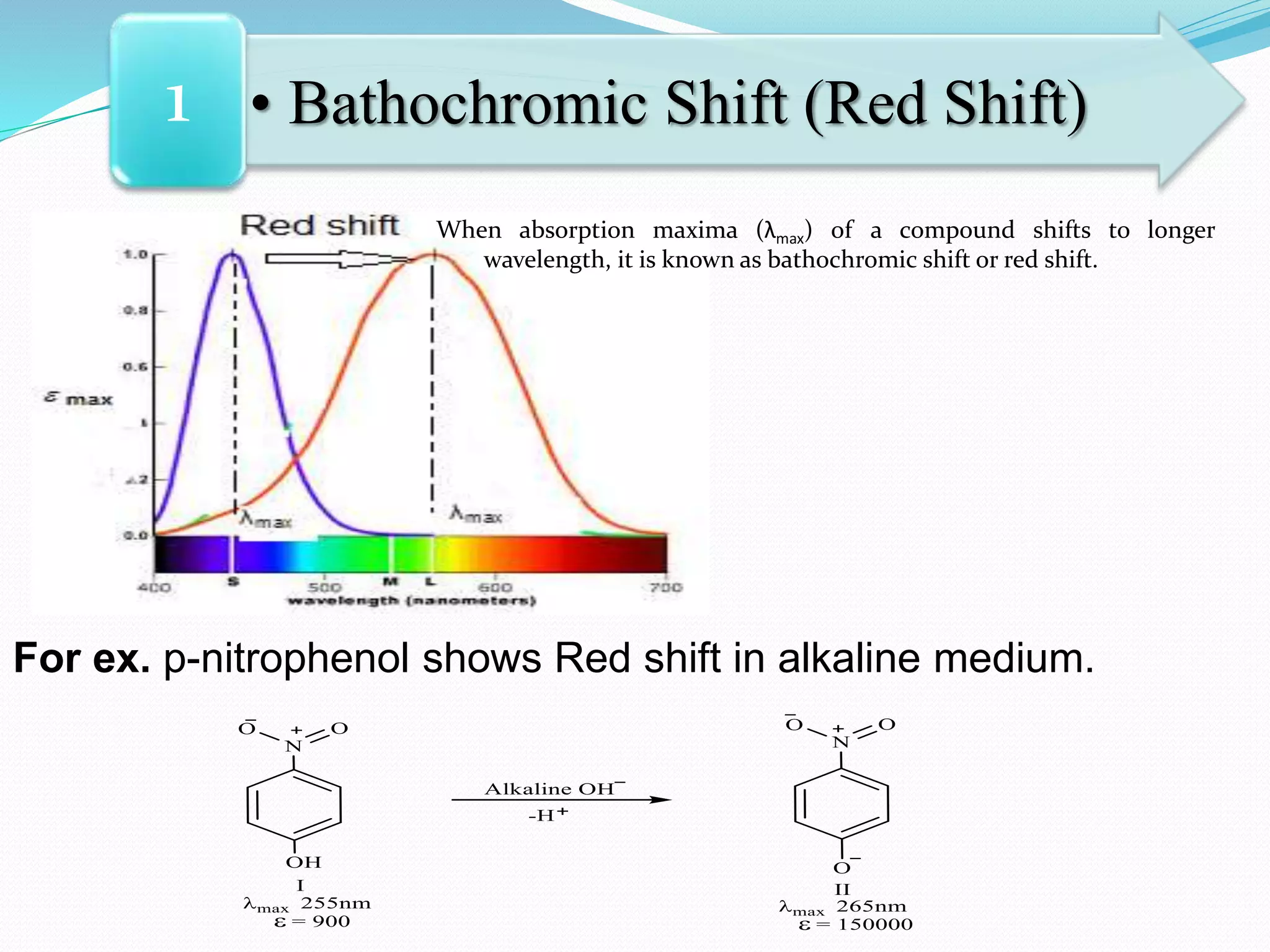

![• Bathochromic Shift (Red Shift)1
The bathochromic effect or Red shift is produced due to:
1) Presence of auxochrome (increase in lone pairs)
2) Increase in conjugation
3) Increase in polarity of solvent
4) Any effect which produces additional lone pair and
negative charge on hetero donor atom causing
greater resonance delocalization of lone pair.
[Note: The n → π* transition for carbonyl compounds shows Bathochromic shift
when the polarity of solvent decreases.]](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-109-2048.jpg)
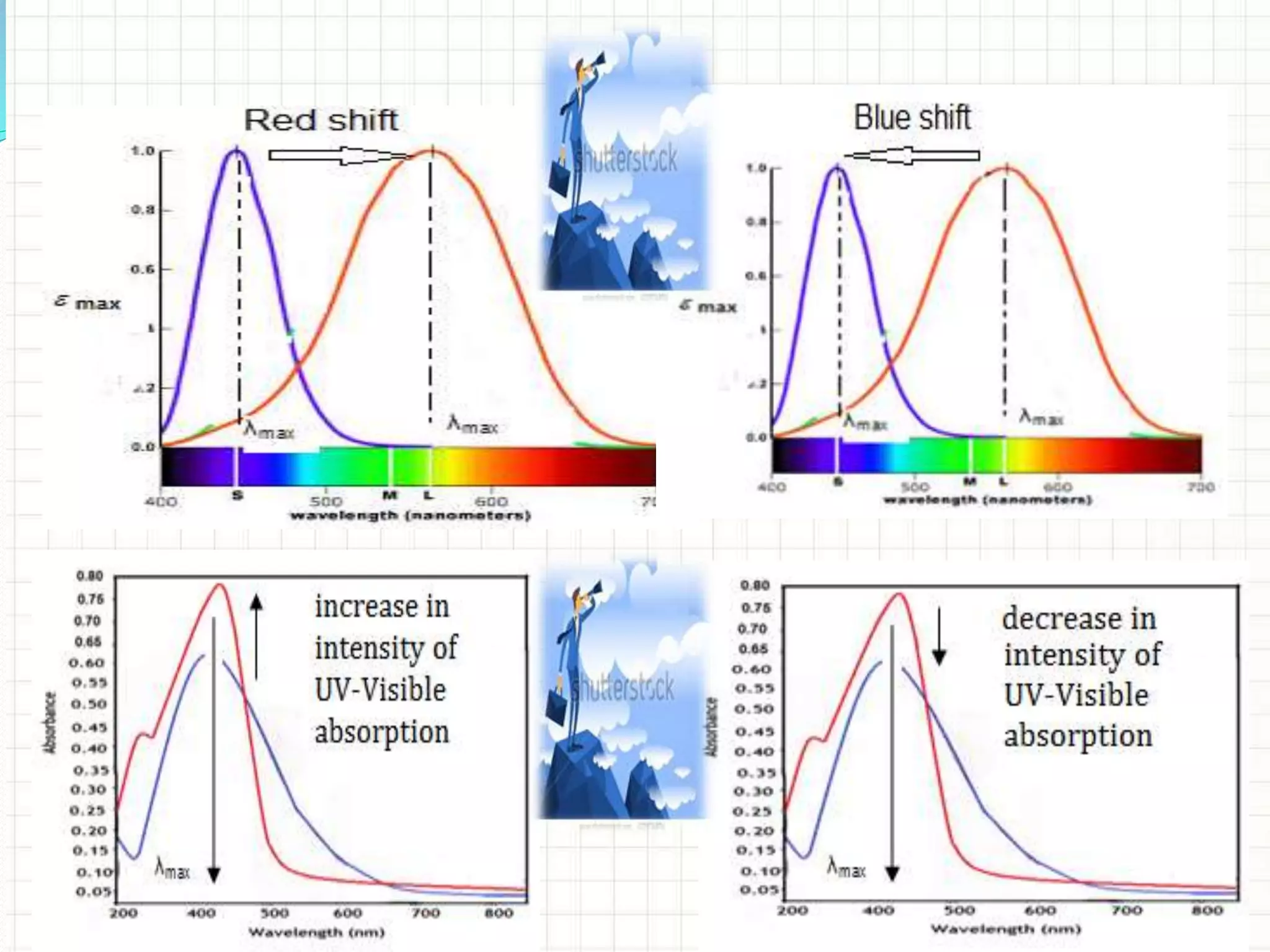
![The polar solvent increases π → π* λmax (red shift) and
decreases n → π* and n → σ* λmax (blue shift).
[Note: The n → π* transition for carbonyl
compounds shows Bathochromic shift when the
polarity of solvent decreases.]](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-110-2048.jpg)


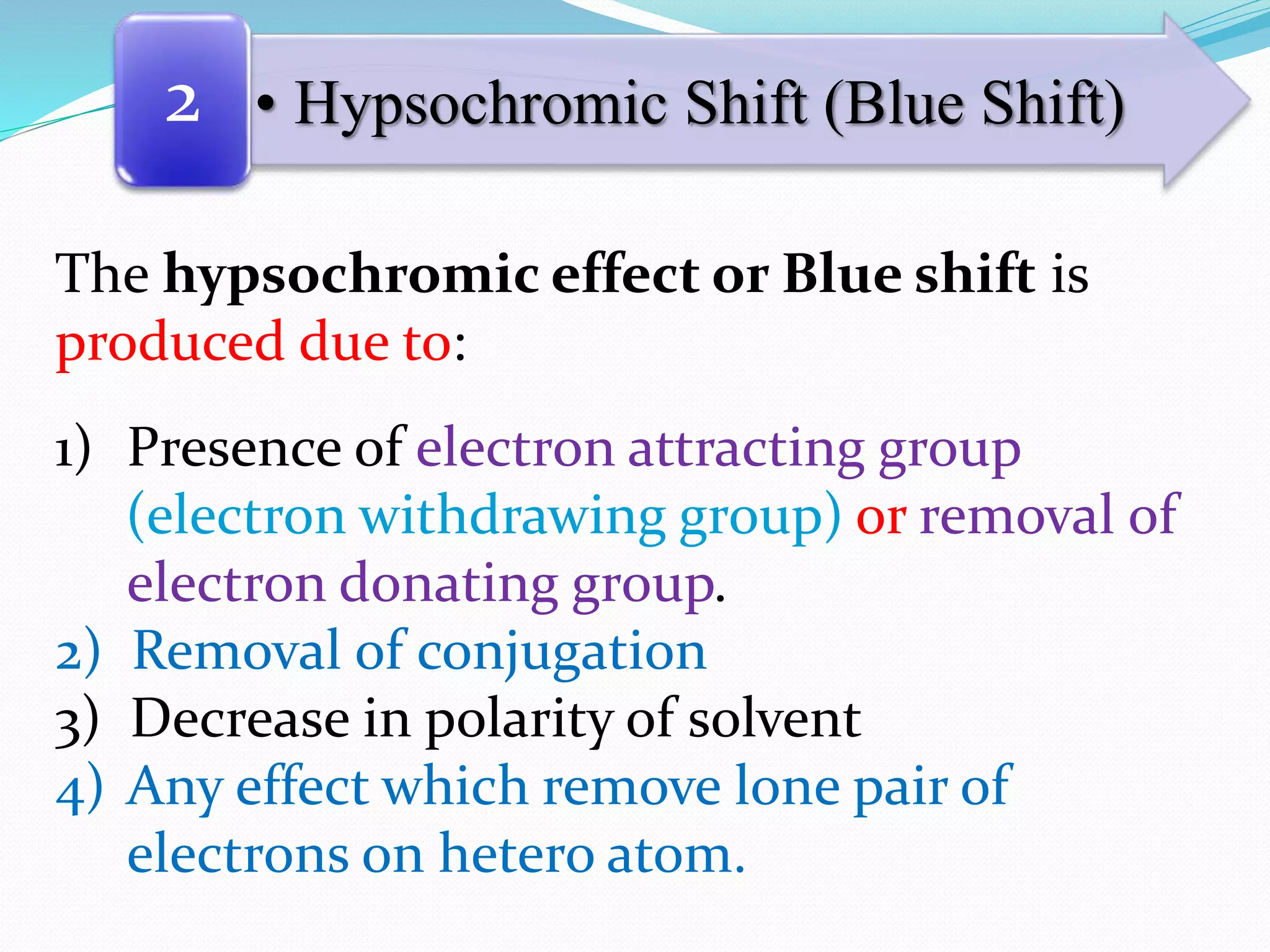


![[Note that:
(ii) Molecules with non-bonded electrons (n) are able
to interact with hydrogen bonding solvents to a greater
extent in the ground state than in their excited state.
As a result the n → π* transition absorption will shift
toward shorter wavelength as the hydrogen bonding
ability of the solvent increases. A shift toward shorter
wavelength is called a hypsochromic effect.]](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-117-2048.jpg)






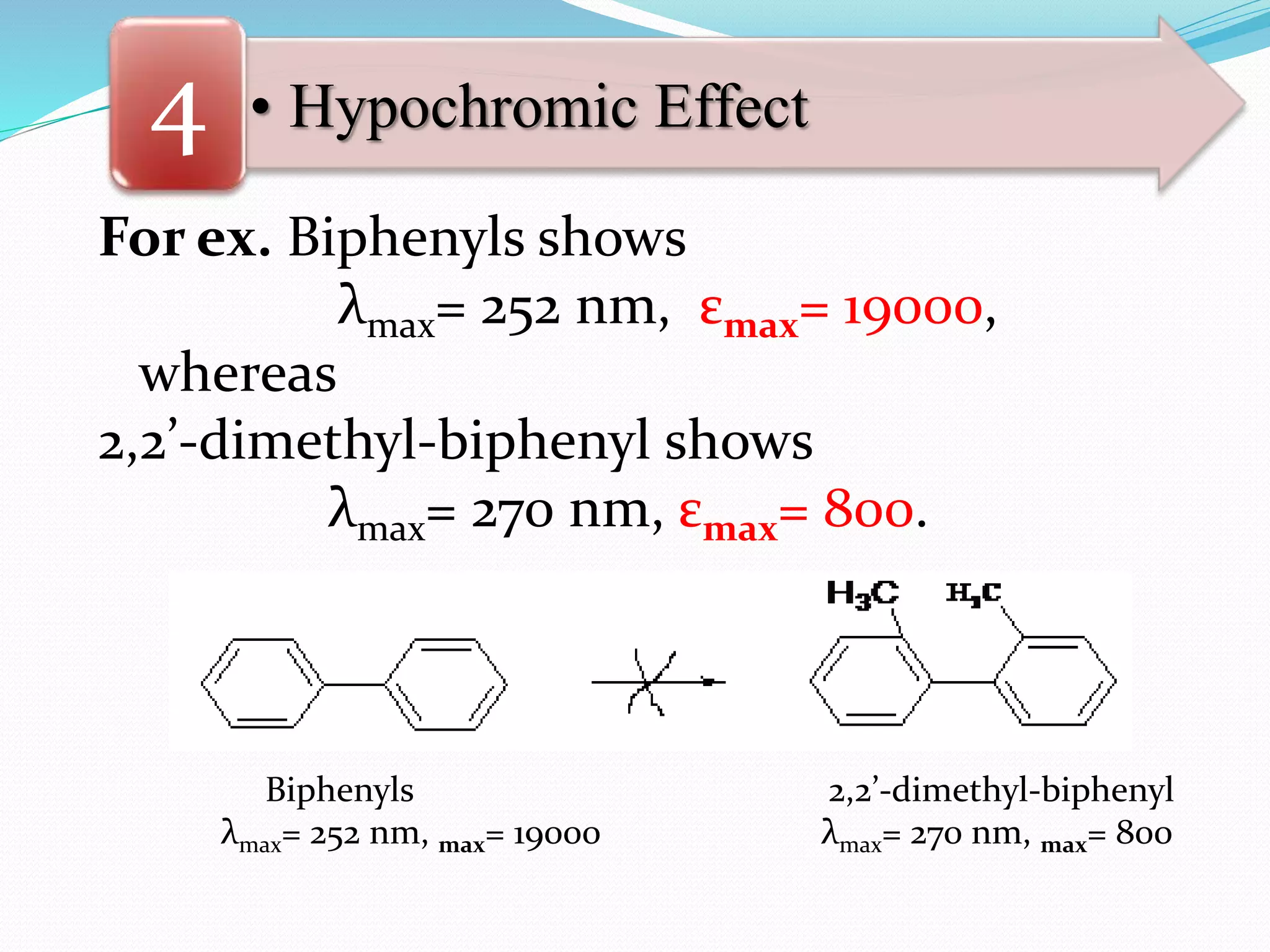
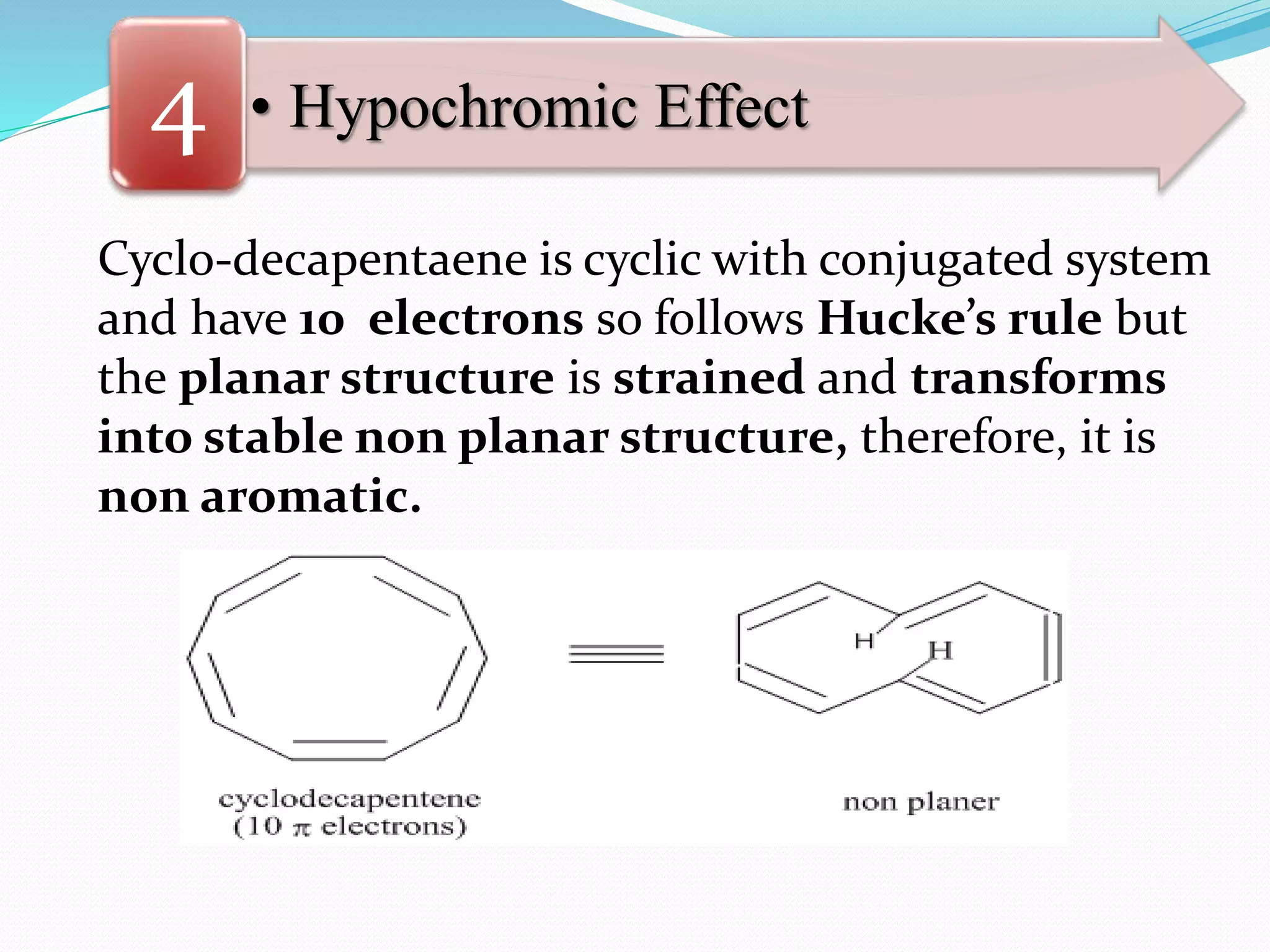
![• Hypochromic Effect4
Biphenyls 2,2’-dimethyl-biphenyl
λmax= 252 nm, ε max= 19000 λmax= 270 nm, ε max= 800
[Note that:
The decrease of 18,200 in the value εmax of 2,2’-
dimethyl-biphenyl is due to the hypochromic
effect of the methyl groups which distort the
chromophore by forcing the rings out of
co-planarity resulting in the loss of conjugation.]](https://image.slidesharecdn.com/uv-visiblespectroscopybydr-200306185221/75/Uv-visible-spectroscopy-by-dr-pamod-r-padole-126-2048.jpg)