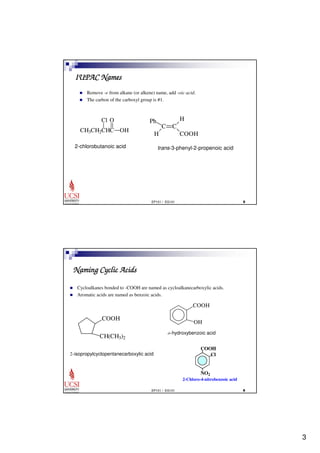
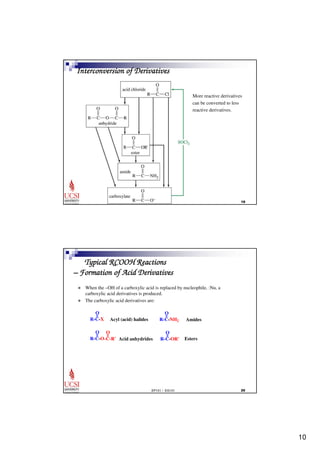
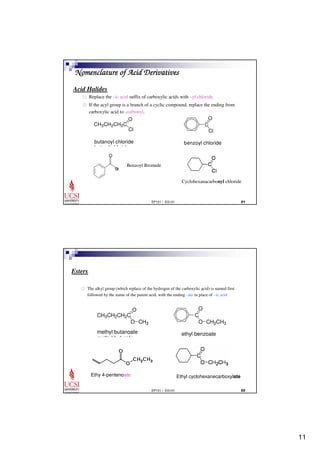
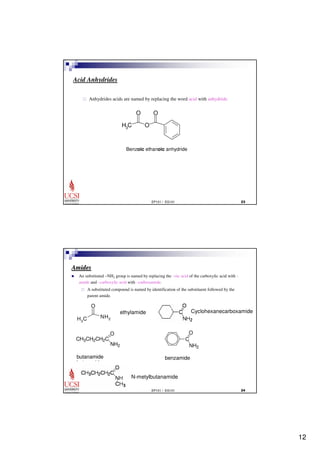
This document provides information about carboxylic acids and their derivatives. It begins by stating the learning outcomes, which are to provide nomenclature of carboxylic acids and derivatives, describe physical properties of carboxylic acids, and explain the synthesis and reactions of carboxylic acids and derivatives. The document then discusses the structure, naming rules, physical properties, acid strength, and synthesis methods of carboxylic acids. It also explains the nomenclature and reactions of common carboxylic acid derivatives like esters, acid halides, anhydrides, and amides.