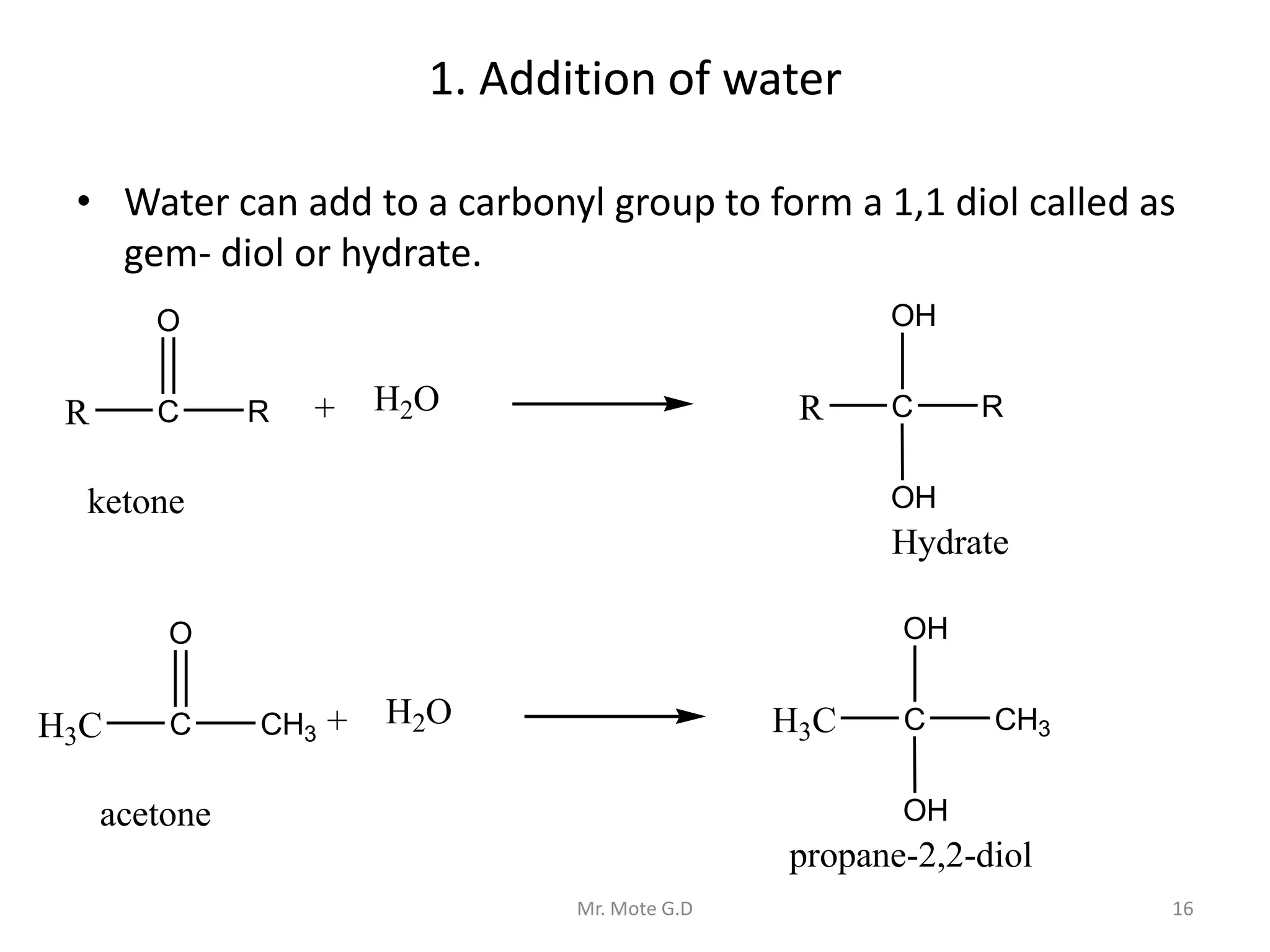
The document outlines various methods for the preparation of aldehydes and ketones, including oxidation of alcohols, catalytic dehydrogenation, hydration of alkynes, and reduction of acid chlorides, among others. It also discusses the reactions involving aldehydes and ketones such as nucleophilic addition reactions, reduction, oxidation, and several condensation reactions. Furthermore, it includes identification tests for aldehydes and ketones, providing details on distinct reagents and the expected outcomes.






































![Identification test for aldehyde and ketone
Sr.
No
Name of test Aldehyde Ketone
1 DNP test
DNP solution: phenyl hydrazine in 5M
hydrochloric acid .
Red or orange
ppt
Red or orange
ppt
2 Tollen’s test :dissolve *1 gm of silver nitrate in
10 ml of water] ( solution A ) and [1 gm of
NaOH in 10 ml of water] (solution B ) when
the reagent is required mix equal volumes 1 ml
from A & B in a clean test tube and
add dilute ammonia solution drop by drop until
the silver oxide is just dissolve
Red ppt on
heating of
sample
solution with
Tollen’s
reagent
Red ppt
heating of
sample
solution with
Tollen’s
reagent
3 Fehling’s reagent
Fehling Reagent A: Copper sulphate and
sulphuric acid in water.
Fehling reagent B: Sodium hydroxide and
Sodium potassium tartarate in water
Red ppt with
Fehling
reagent A and
B by heating
Red ppt with
Fehling
reagent A and
B by heating
4 Sodium nitroprusside no red color
with sodium
nitropruside
Red color
with sodium
nitropruside](https://image.slidesharecdn.com/aldehydeandketone-190130150934/75/Aldehyde-and-ketone-40-2048.jpg)
