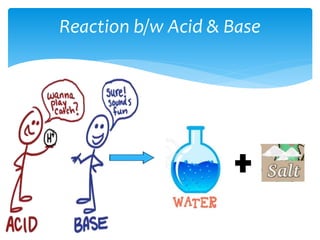
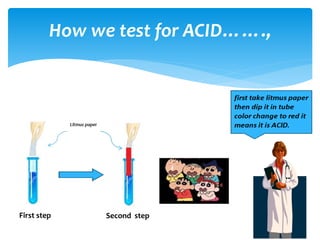
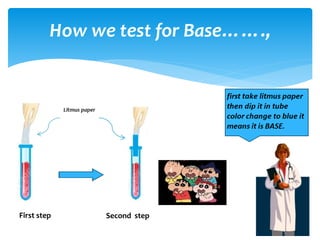
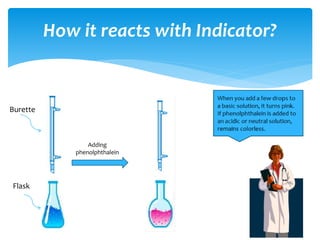
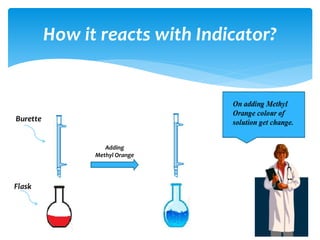
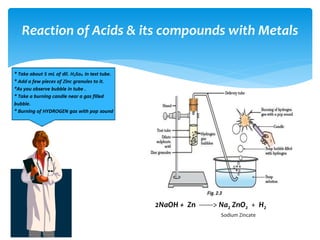
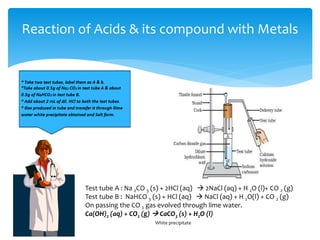
The document is a presentation on acids, bases, and salts, detailing their properties, reactions, and significance in everyday life. It explains testing methods for acids and bases, their reactions with indicators, and their effects on soil pH, digestion, and dental health. Additionally, it covers the formation of salts, including the differences between strong and weak acids and bases, as well as applications of various chemical compounds.