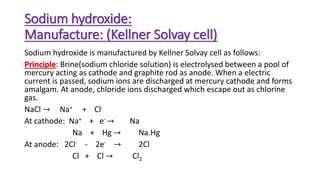
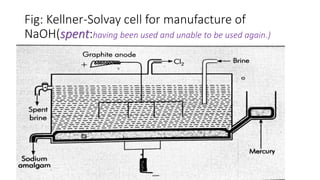
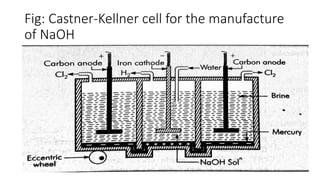
Sodium hydroxide is manufactured through the Kellner-Solvay process and Castner-Kellner cell process. In the Kellner-Solvay process, brine is electrolyzed between a mercury cathode and graphite anode, producing sodium amalgam at the cathode which is later treated with water to produce sodium hydroxide and regenerate the mercury. In the Castner-Kellner cell process, a vessel is divided into compartments where sodium ions are reduced at the mercury cathode to form sodium amalgam, which then reacts with hydroxyl ions in another compartment to form sodium hydroxide. Sodium hydroxide is a white, highly alkaline and water-soluble solid with various industrial and laboratory uses such