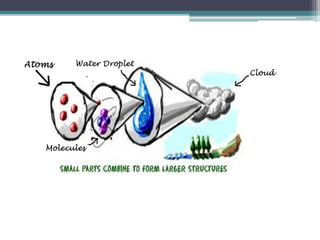
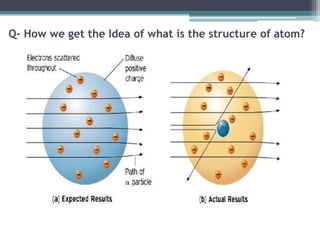
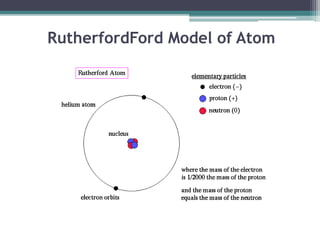
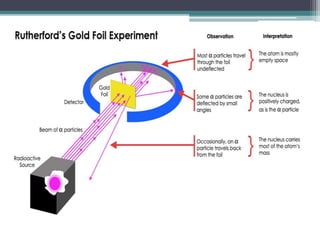
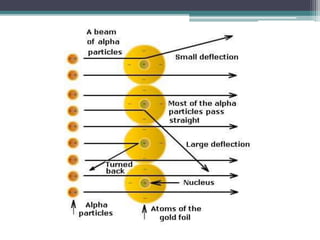
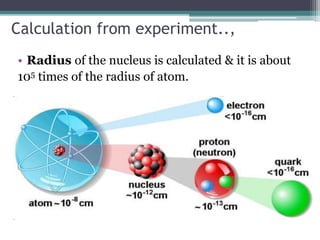
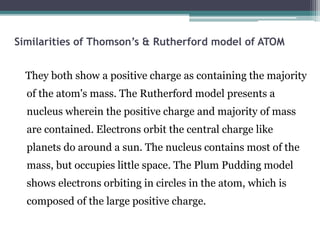
The document discusses the structure of the atom based on Rutherford's experiment, highlighting that atoms are tiny, indivisible particles. Rutherford's experiment with gold foil revealed that most of the atom is empty space, with a dense nucleus containing positive charge and mass. It contrasts this with Thomson's plum pudding model, emphasizing the significant shifts in understanding atomic structure brought about by Rutherford's findings.