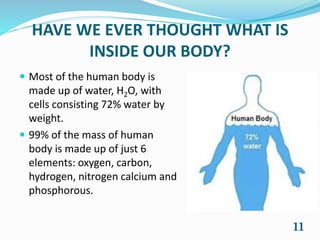
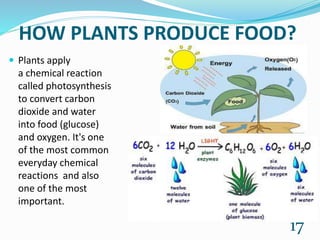
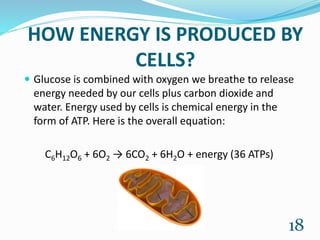
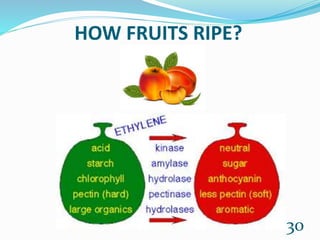
Chemistry is present in everyday life through foods, air, cleaning products, emotions, and all objects. Some common examples of chemistry include water, table salt, nail polish remover, table sugar, battery acid, and vinegar. Many chemical reactions also occur in daily life through photosynthesis, cellular respiration, rusting of iron, digestion, combustion, soap cleaning, baking soda and vinegar reactions, carbonation, acid rain, ant bites, fruit ripening, and flowers. Chemistry plays a vital role in providing necessities like food, medicine, fuels, and materials through various chemical processes and compounds.