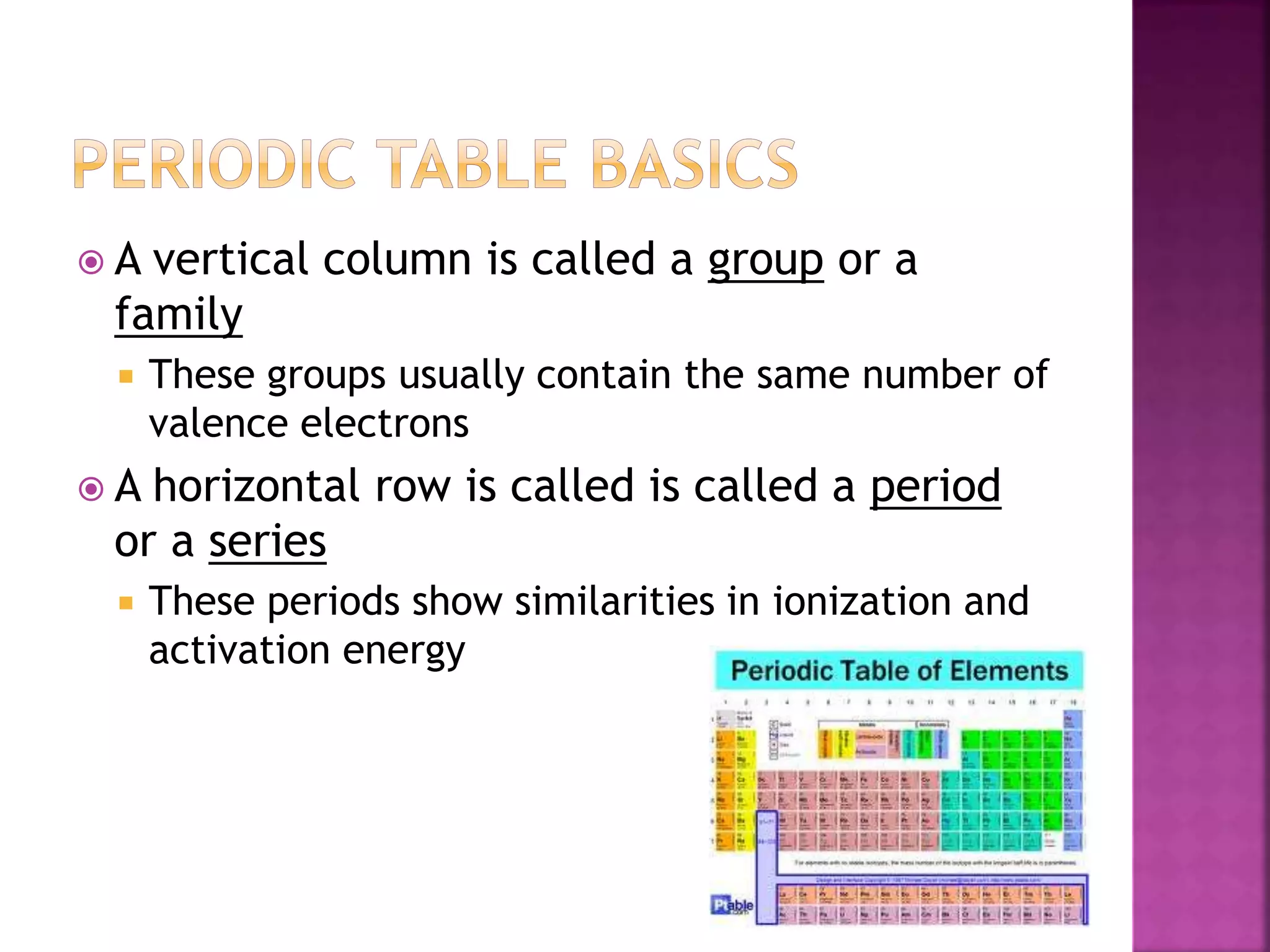
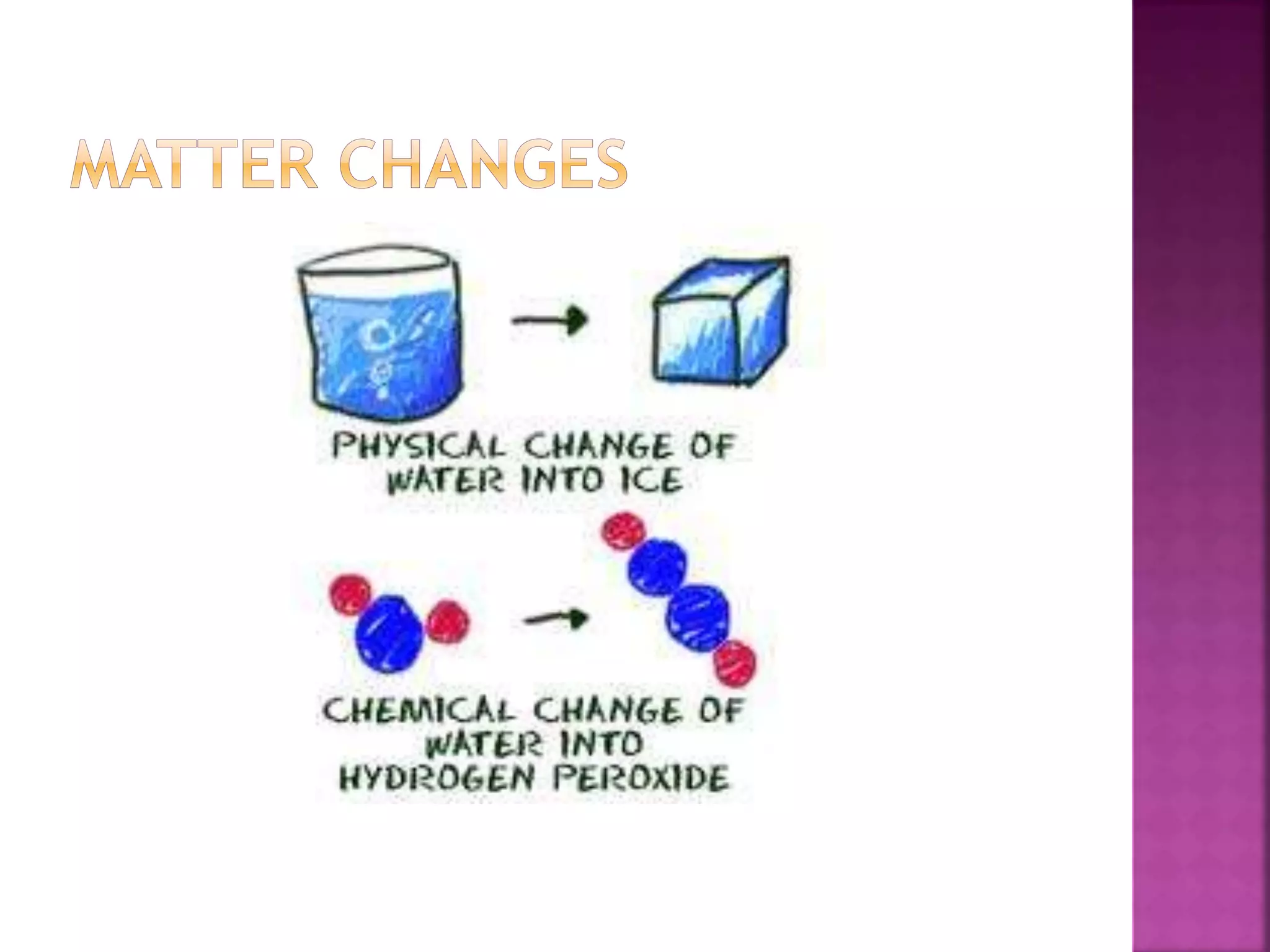
This document provides an overview of basic chemistry concepts. It discusses that chemistry is the study of matter and its properties, and that all living things are made up of chemicals and chemical processes. The three main states of matter are solids, liquids, and gases. Key concepts covered include elements, atoms, the periodic table, chemical bonds including ionic and covalent bonds, and the four main types of macromolecules that make up living things: carbohydrates, proteins, lipids, and nucleic acids. Chemical reactions such as synthesis, decomposition, and exchange reactions are also briefly discussed.