Embed presentation
Download as PDF, PPTX
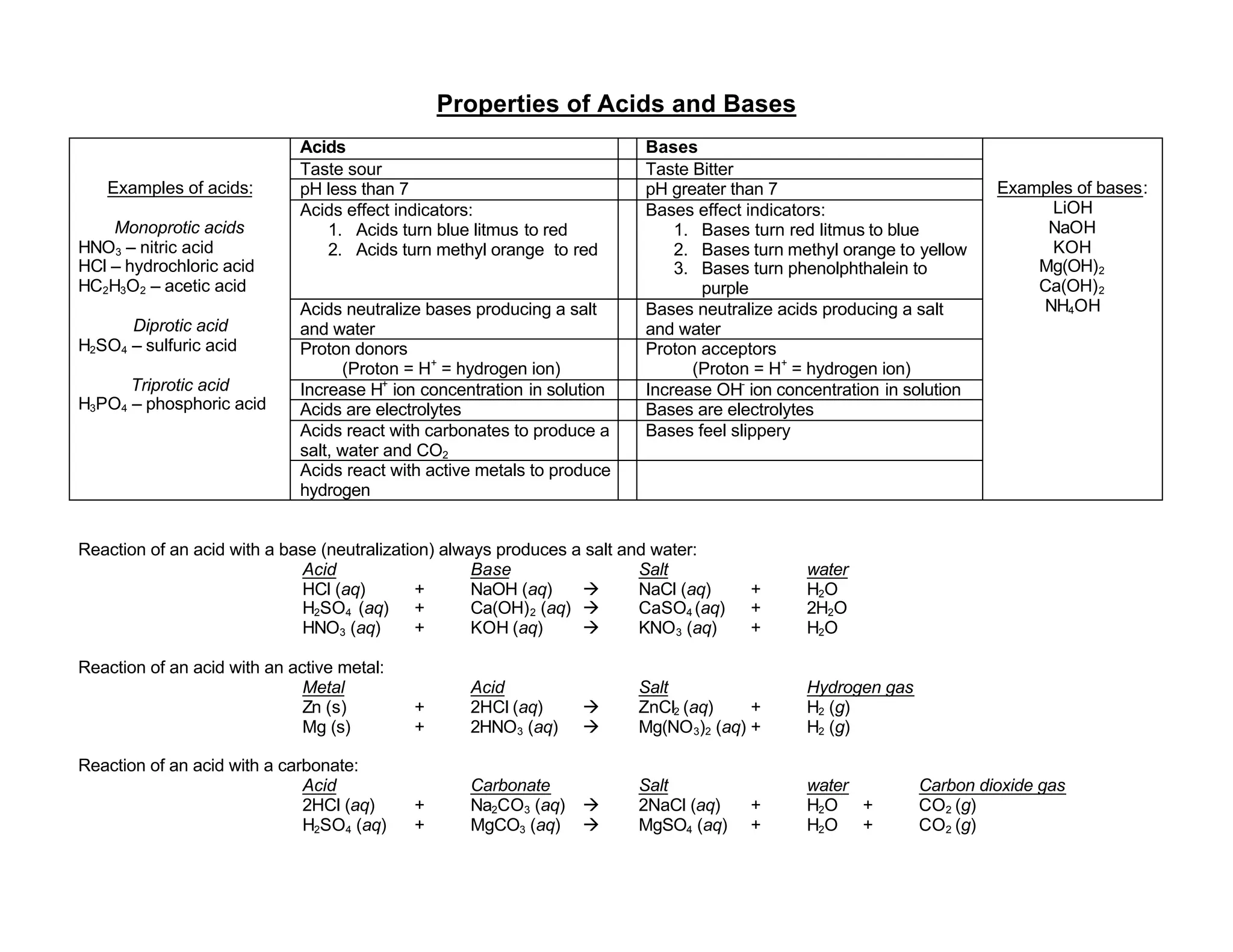

Acids have a sour taste, are electrolytes, turn indicators red, and have a pH less than 7. They donate protons and can neutralize bases to form salts and water. Bases have a bitter taste, are electrolytes, turn indicators blue or yellow, and have a pH greater than 7. They accept protons and can neutralize acids to form salts and water. Common acids include nitric acid, hydrochloric acid, acetic acid, sulfuric acid, and phosphoric acid. Common bases include lithium hydroxide, sodium hydroxide, potassium hydroxide, magnesium hydroxide, and calcium hydroxide.
