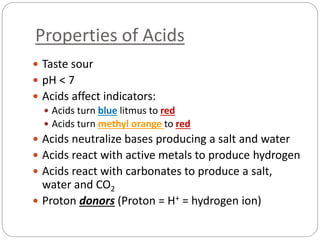
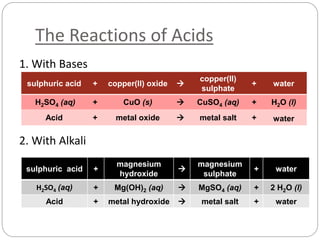
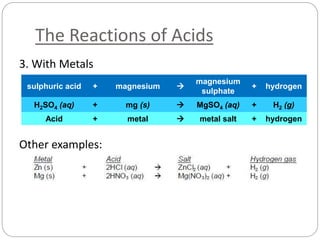
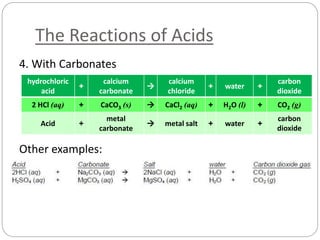
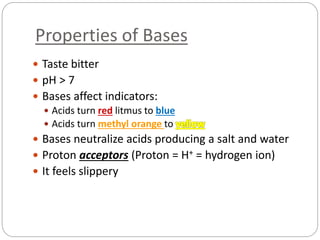
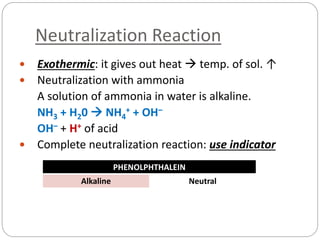
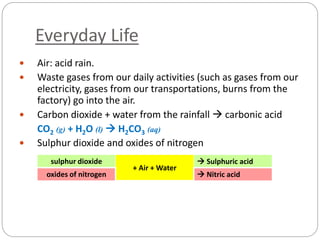
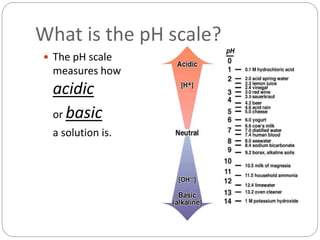
This document discusses acids and bases. It defines acids as substances that produce H+ ions in aqueous solution and bases as substances that produce OH- ions. The document describes the pH scale for measuring acidity and alkalinity. It provides examples of the characteristic reactions of acids with metals, bases, and carbonates. It also discusses the importance of controlling acidity in the environment and describes how acids and bases are encountered in everyday life such as in soil, water, and air.


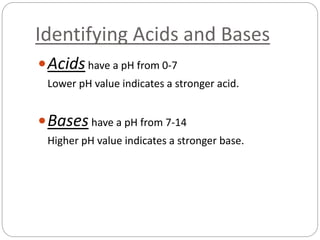
![Definitions of Acids and Bases
An acid is a substance that breaks into [H+] ions in
an aqueous solution.
A Base (alkaline) is a substance that breaks into
[OH–] ions in an aqueous solution.
Note: aqueous solution is any solution where H2O
is the solvent.](https://image.slidesharecdn.com/3-151111145253-lva1-app6891/85/Acids-Bases-5-320.jpg)