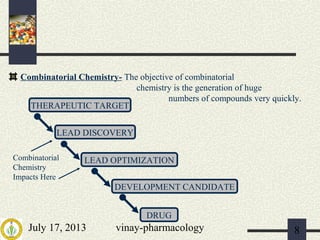
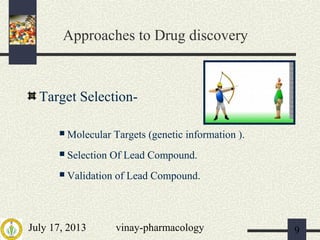
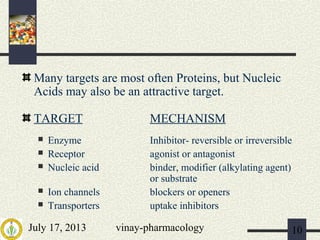
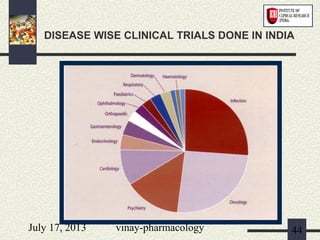
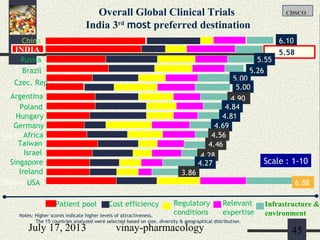
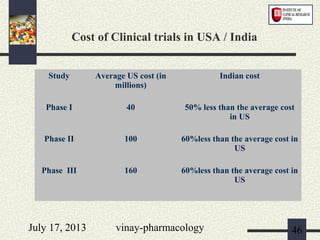
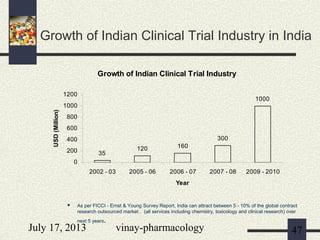
The document discusses various aspects of drug discovery and development, including:
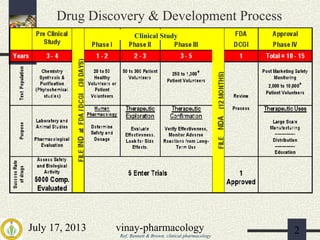
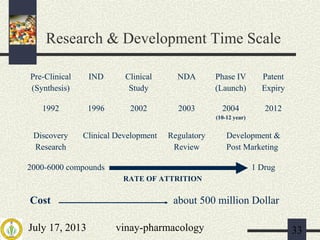
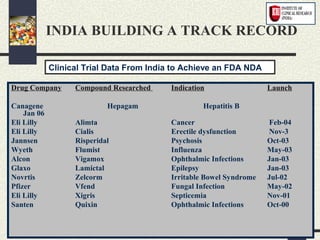
1) The drug development process involves pre-clinical and clinical trials that are regulated by agencies like DCGI in India and FDA in the US.
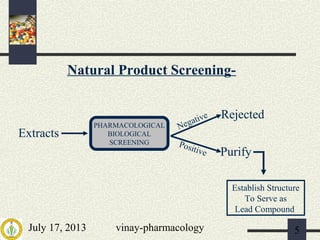
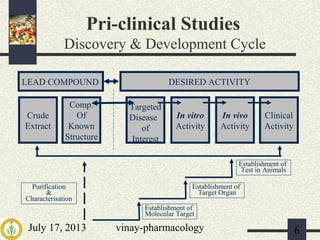
2) Pre-clinical trials involve pharmacological, toxicological, and pharmacokinetic testing in animals to establish safety before human trials.
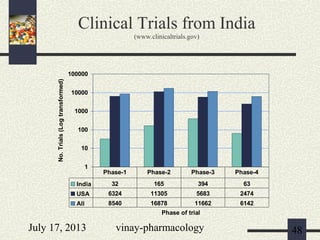
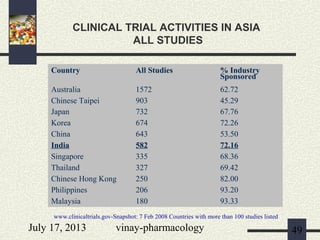
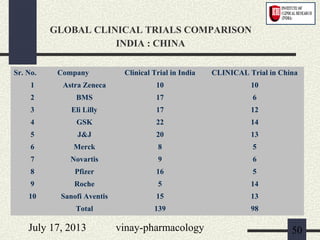
3) Clinical trials have 4 phases - Phase I evaluates safety in healthy volunteers, Phase II explores efficacy in patients, Phase III confirms efficacy and monitors side effects in large patient groups, and Phase IV involves post-marketing surveillance.
The document provides an overview of the drug development pathway and requirements for clinical trials and regulatory approval.