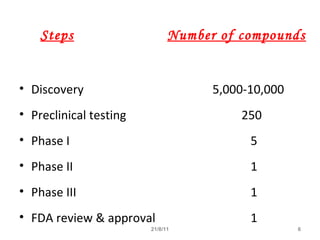
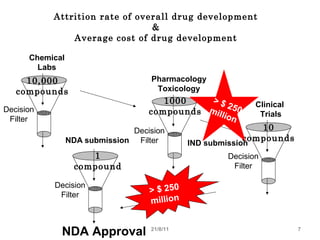
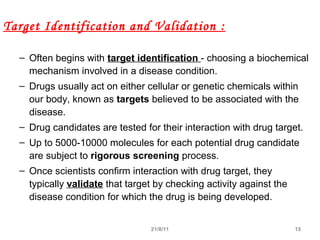
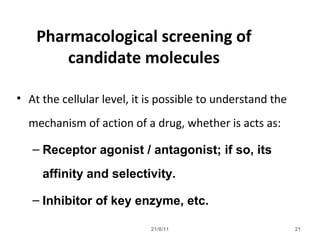
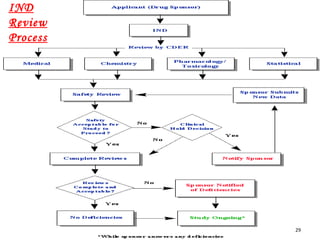
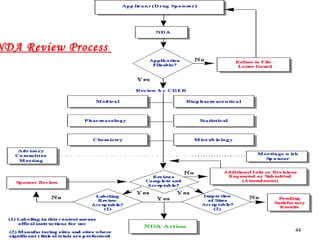
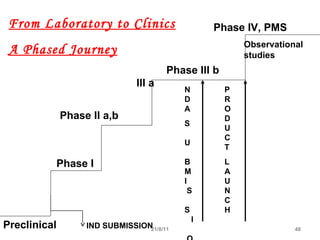
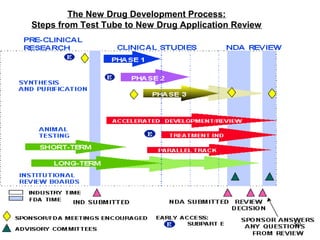
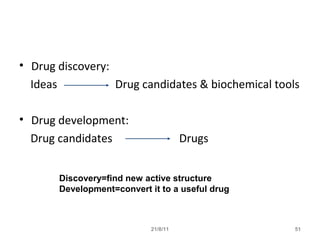
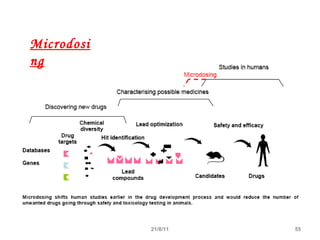
The document provides an overview of the new drug development process, outlining the key steps from discovery and preclinical testing to clinical trials and regulatory approval. It begins with identifying a target and potential drug candidates, then progresses through preclinical screening and safety testing, IND submission, and formal clinical trials in three phases with increasing numbers of participants. The goal is to demonstrate a drug's safety, efficacy, and appropriate dosage before seeking final regulatory review and approval to market the new drug. The entire process from discovery to approval typically takes 10-12 years and costs over $250 million.