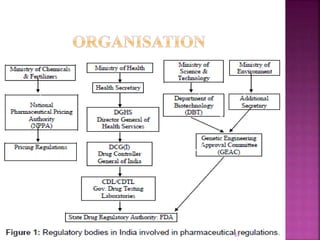
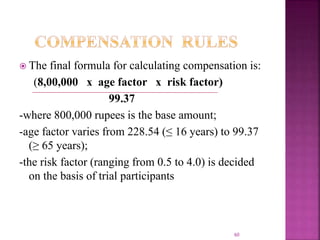
The document outlines the regulatory framework governing clinical trials in India, primarily under Schedule Y and the Drugs and Cosmetics Act. It details requirements for conducting clinical trials, including informed consent, ethical committee responsibilities, and adverse event reporting. Additionally, it describes compensation rules for trial-related injuries and emphasizes adherence to Good Clinical Practice standards.