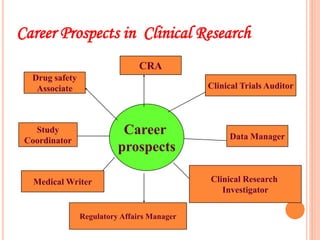
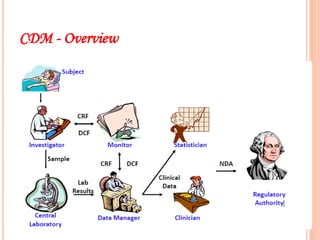
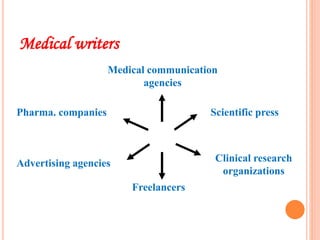
Clinical research involves systematically studying new drugs in human subjects to discover and verify their safety, efficacy, clinical effects, and adverse reactions. Careers in clinical research include clinical research associate, clinical research coordinator, clinical investigator, medical advisor, biostatistician, data manager, clinical trial auditor, medical writer, and clinical research trainer. Clinical research work involves clinical trial processes and operations, clinical data management, medical writing, pharmacovigilance, regulatory affairs, and quality assurance.