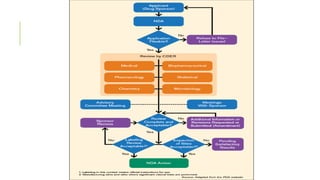
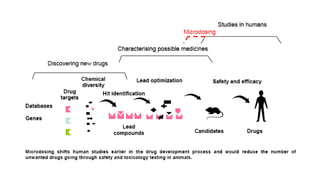
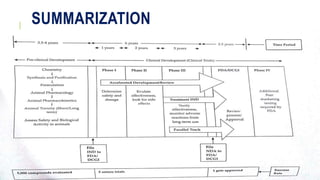
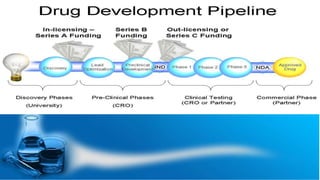
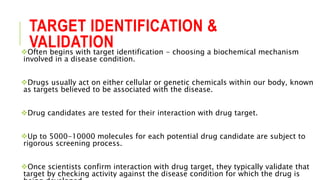
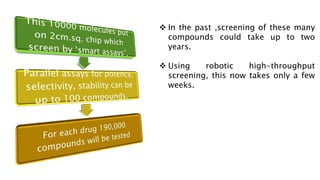
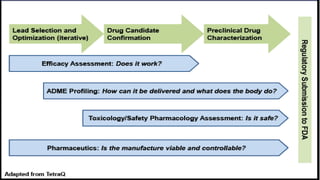
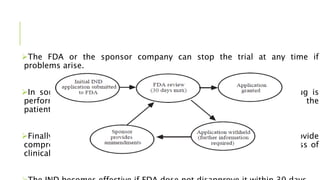
This document discusses the process of new drug development. It begins with basic research to understand disease pathways and identify potential drug targets. Promising compounds are identified through screening and optimized in preclinical testing, which evaluates safety and effectiveness in animals. If preclinical results are satisfactory, an Investigational New Drug Application is submitted to regulators to seek approval for clinical trials in humans. The drug development process is long and rigorous, aiming to bring safe and effective medications to market while meeting regulatory guidelines.













































![ORGANIZATIONAL STRUCTURE FOR A
TYPICAL PHASE III CLINICAL TRIAL
sponsor
Steering
committee
Data
monitoring
committee[DM
C]
Contact Research
organization [CRO]
Data Management
Center
Clinical Study Site Investigators
Data Clarification
Forms (DCFs)
Data Clarification
Forms (DCFs)](https://image.slidesharecdn.com/seminar-newdrugdevelopmnetprocess-190322102008/85/New-drug-development-process-51-320.jpg)