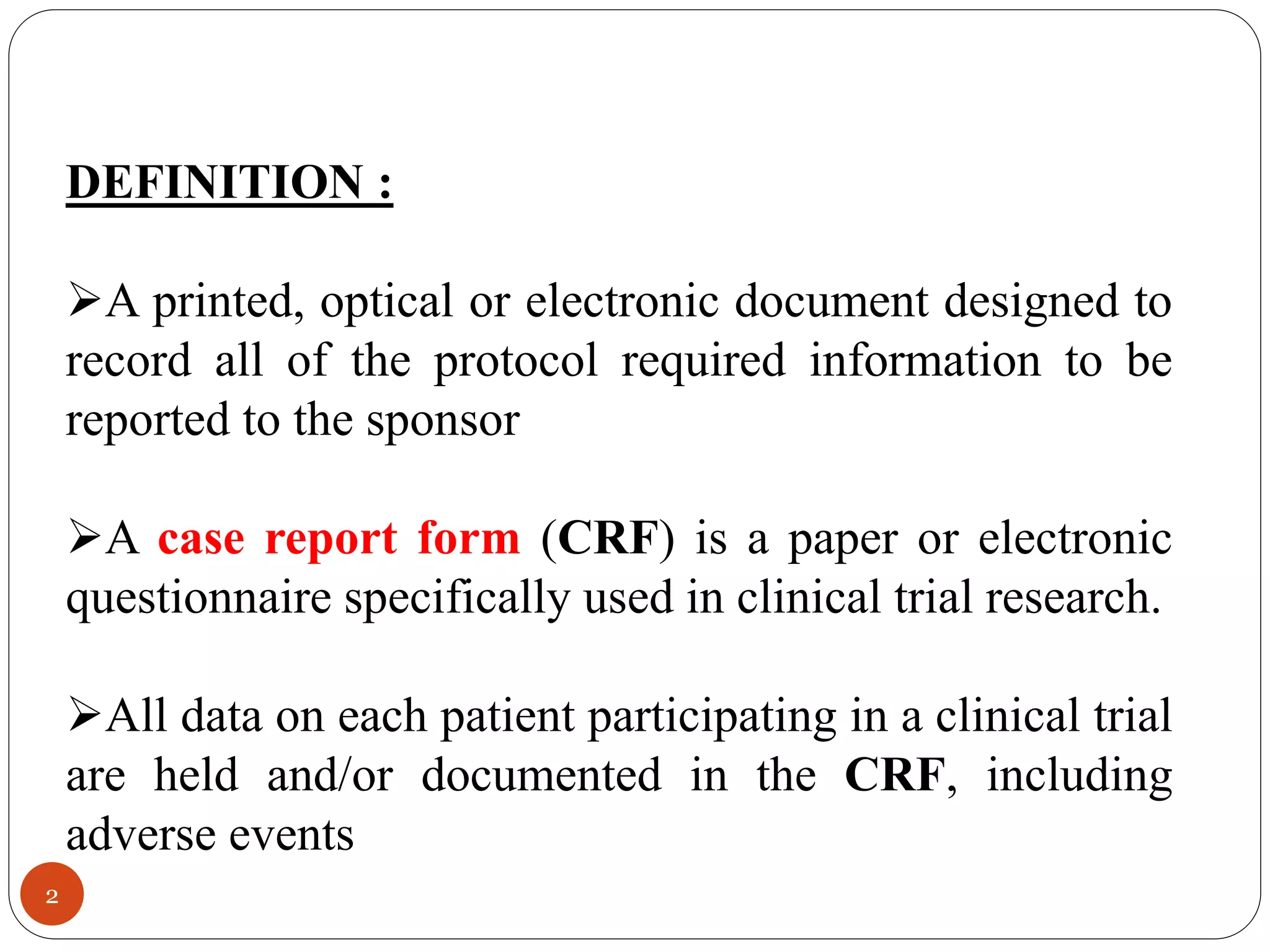
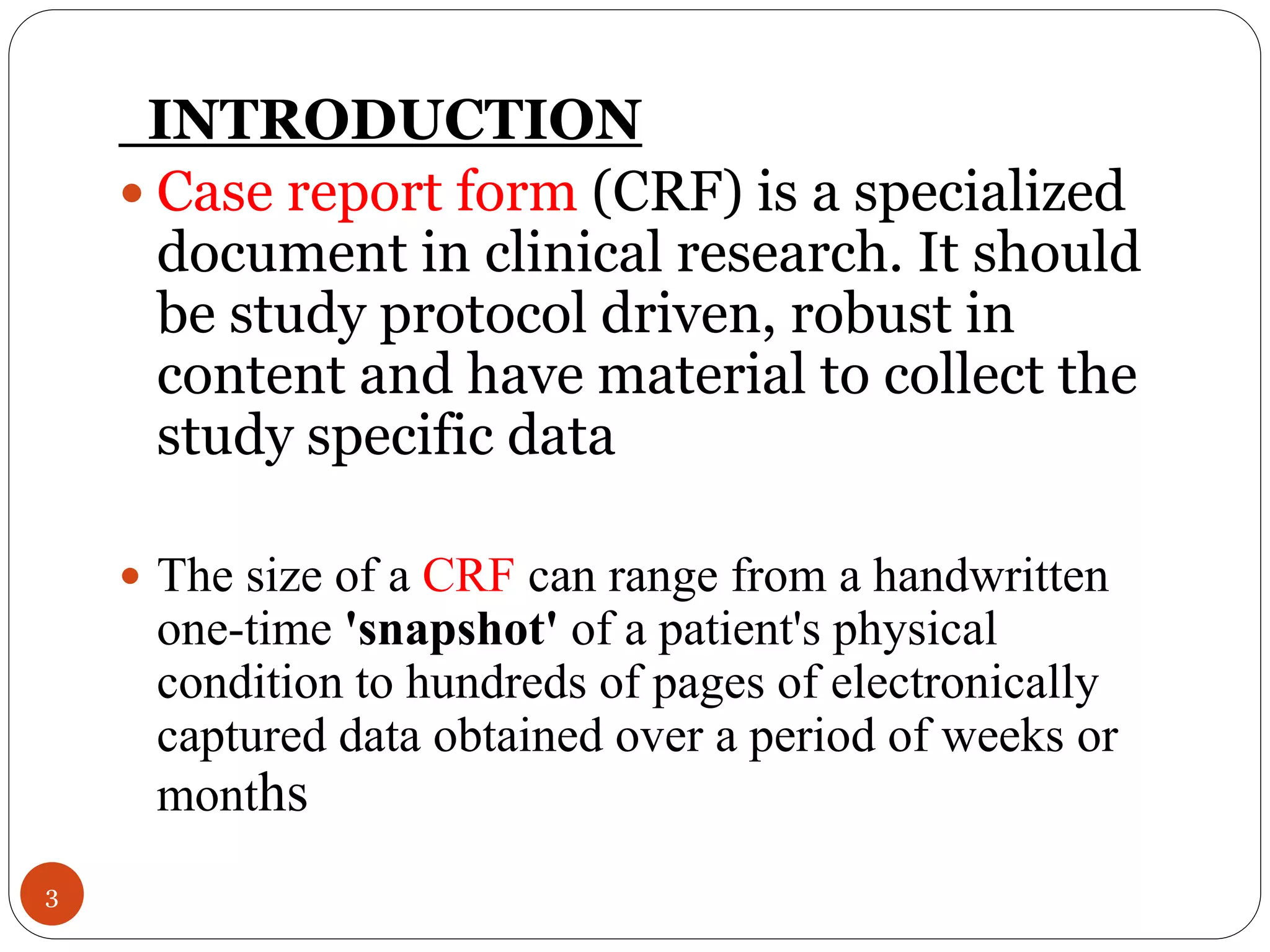
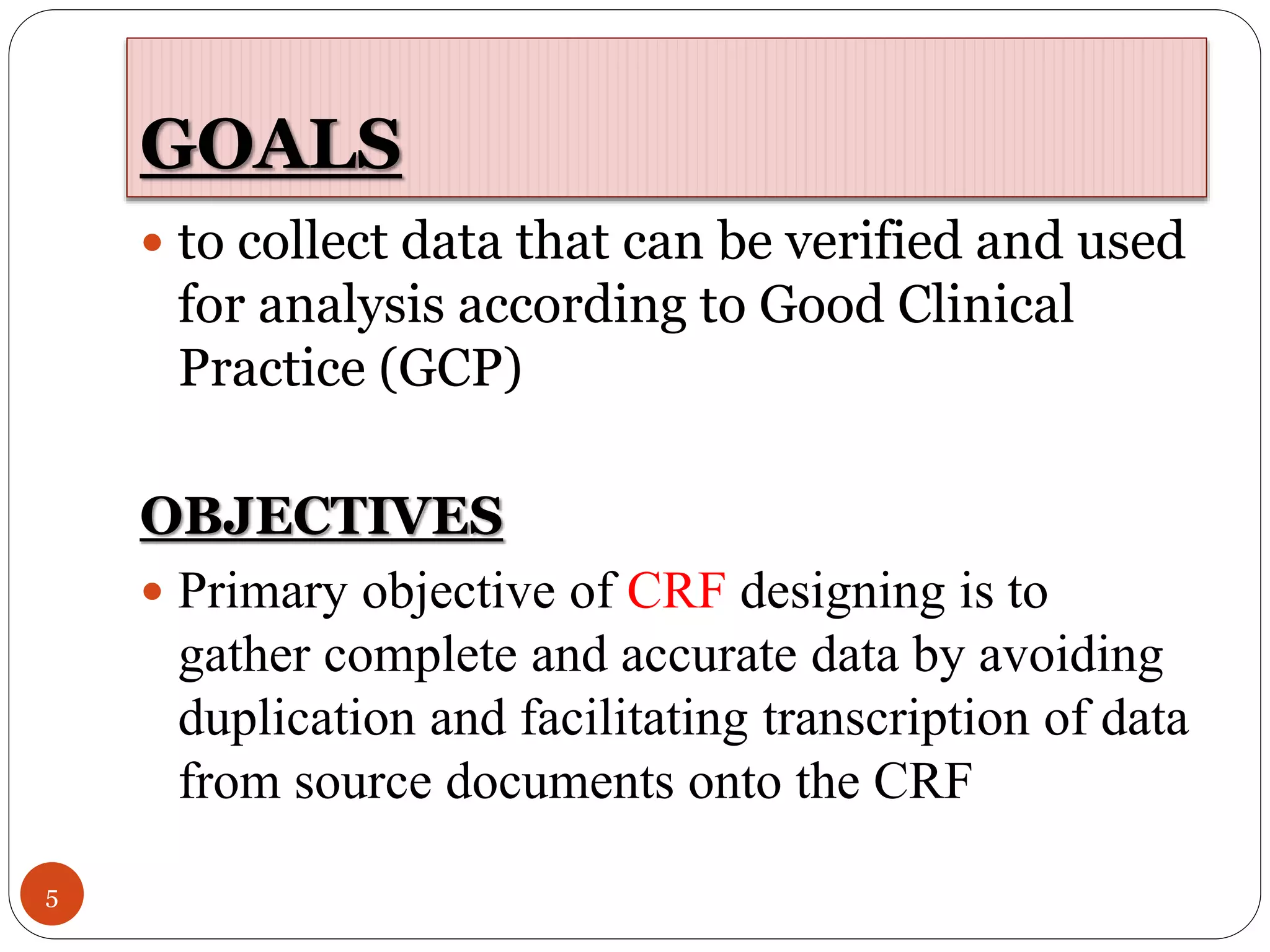
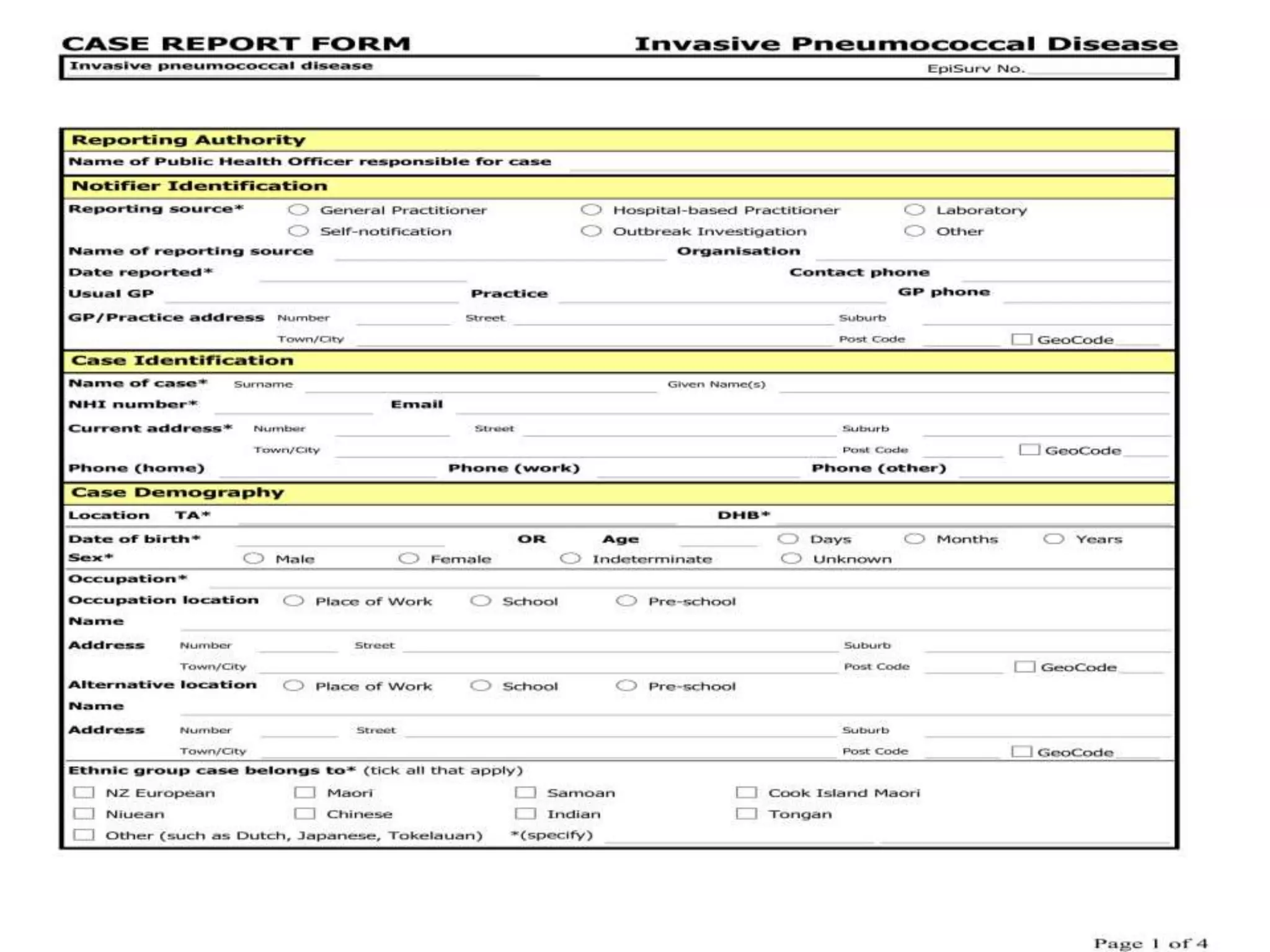
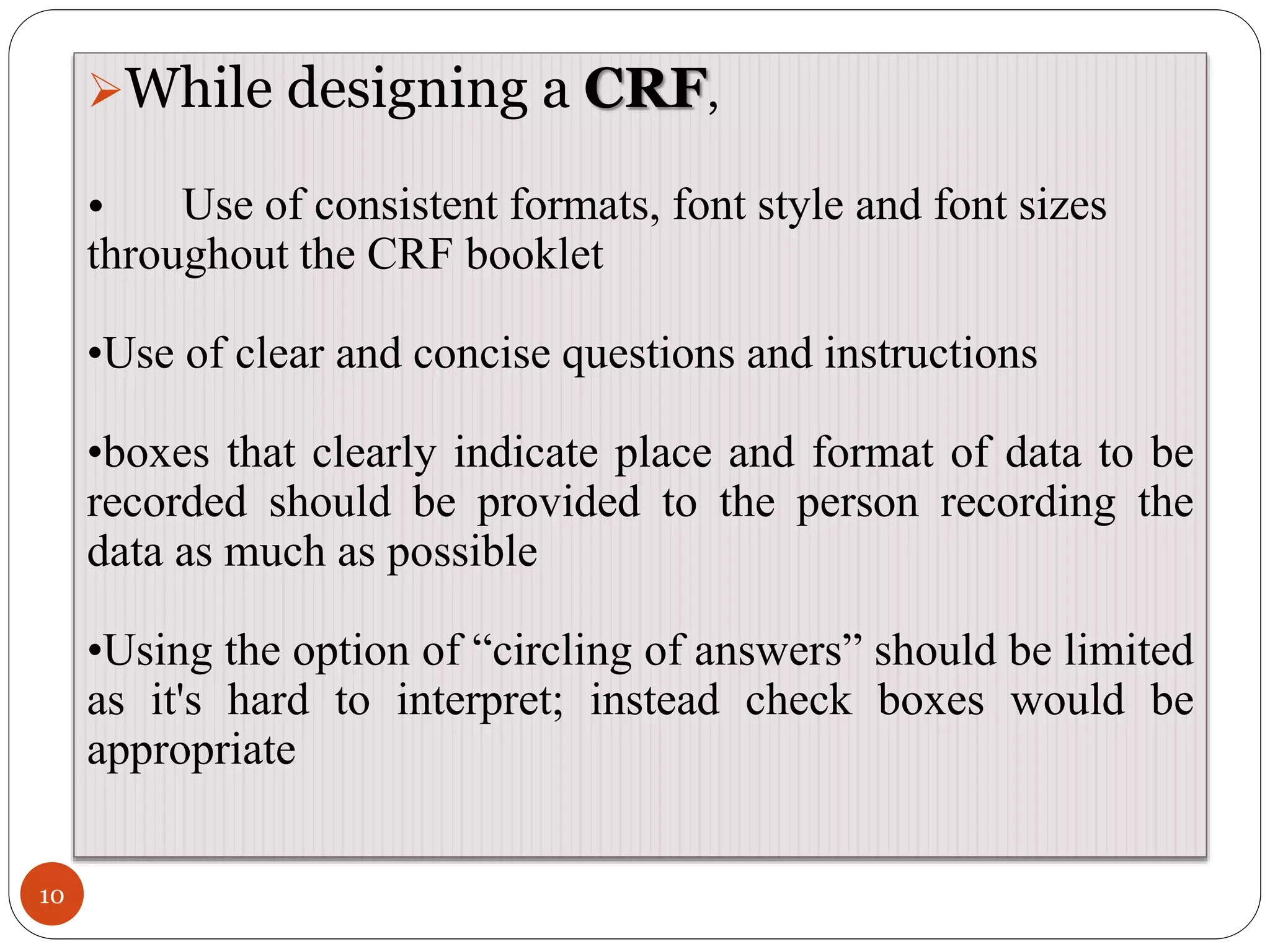
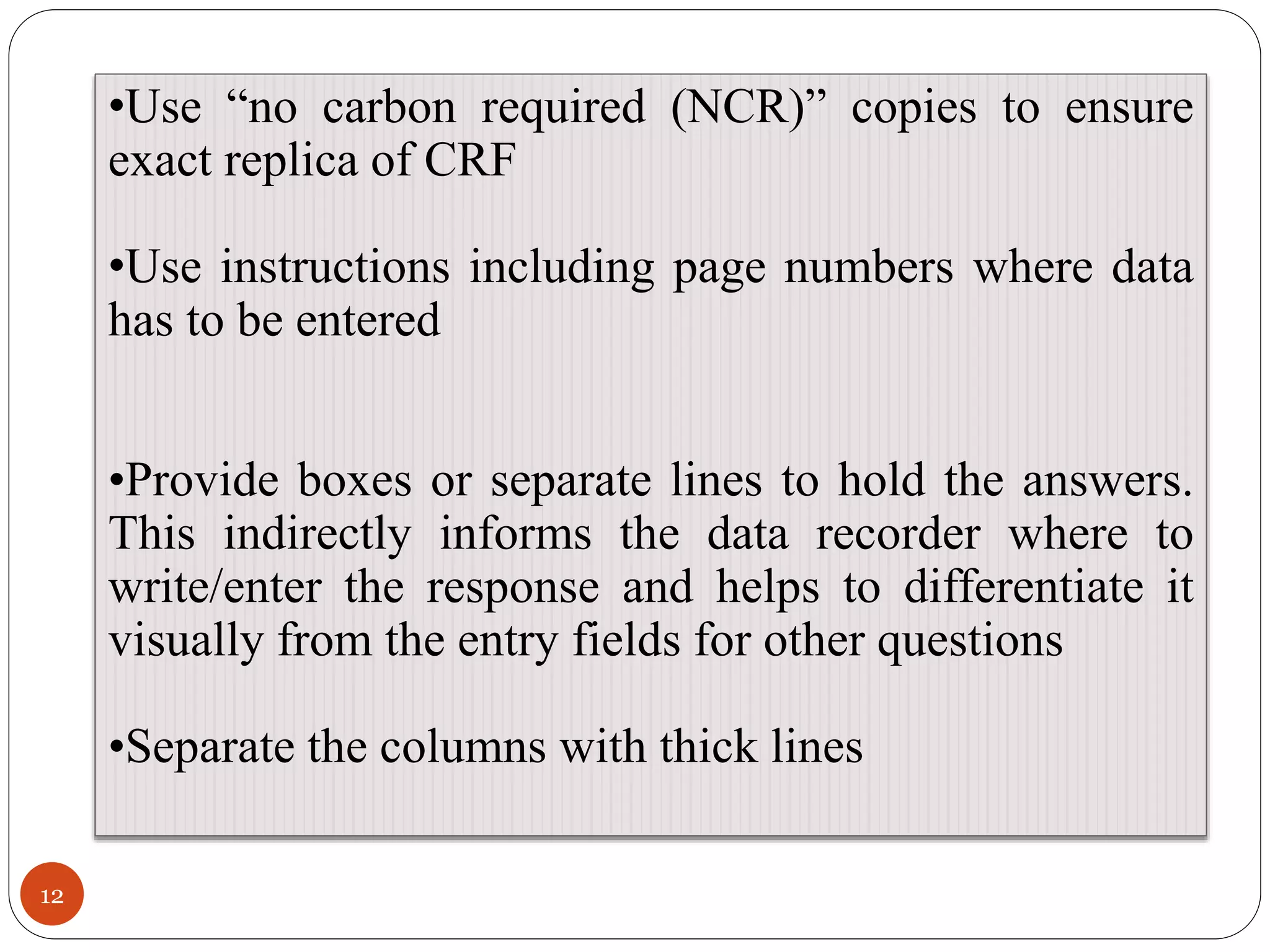
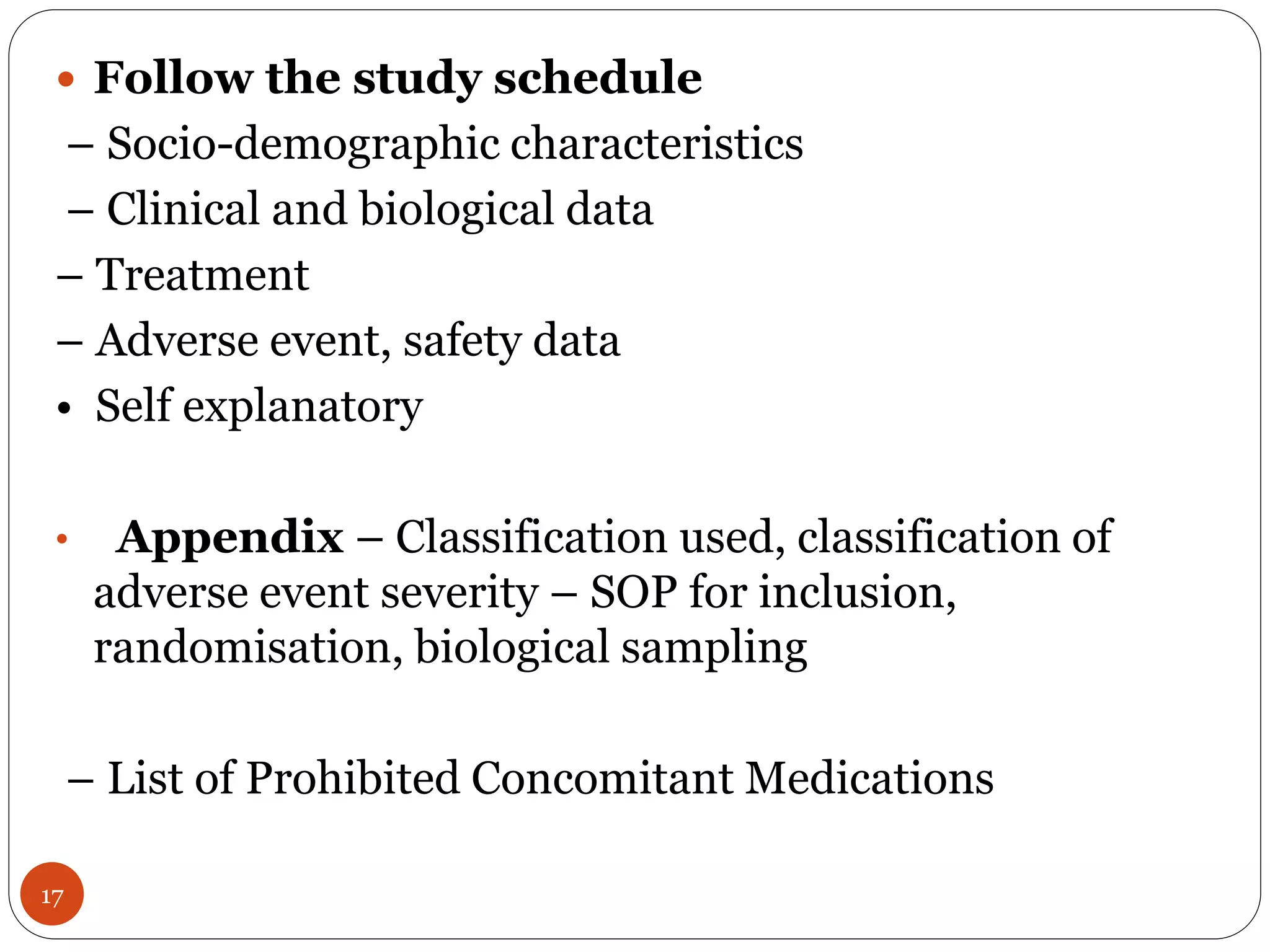
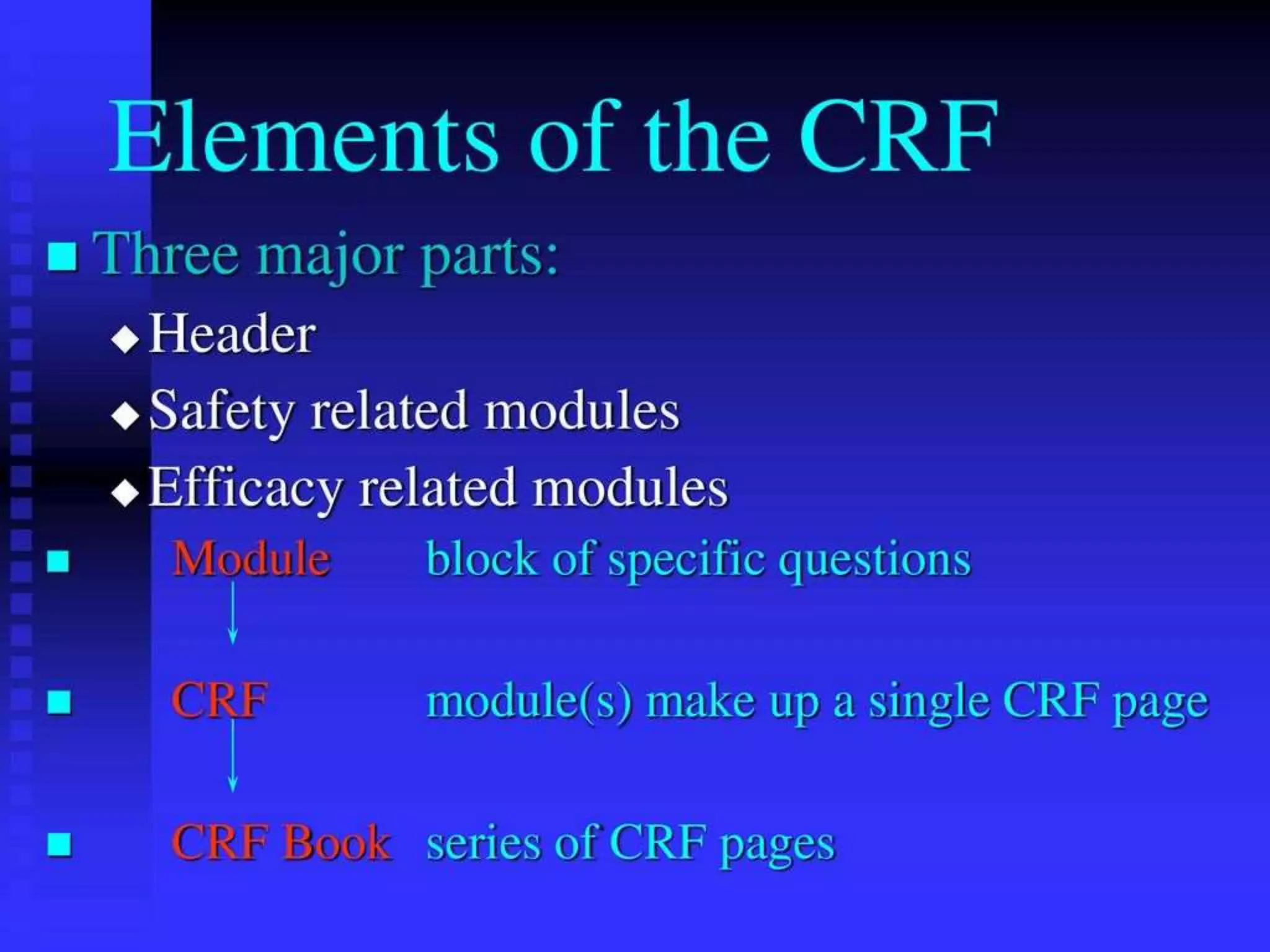
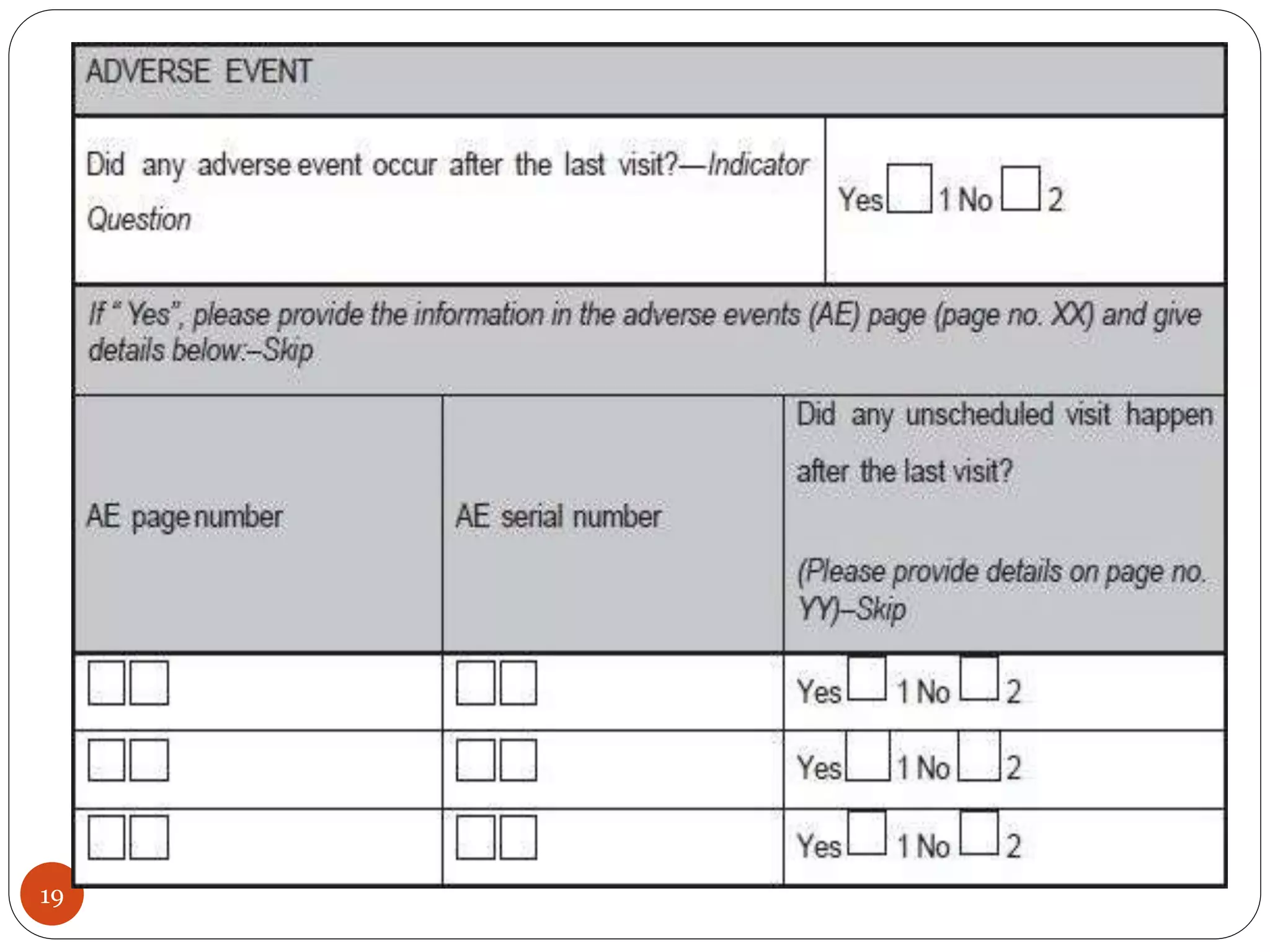
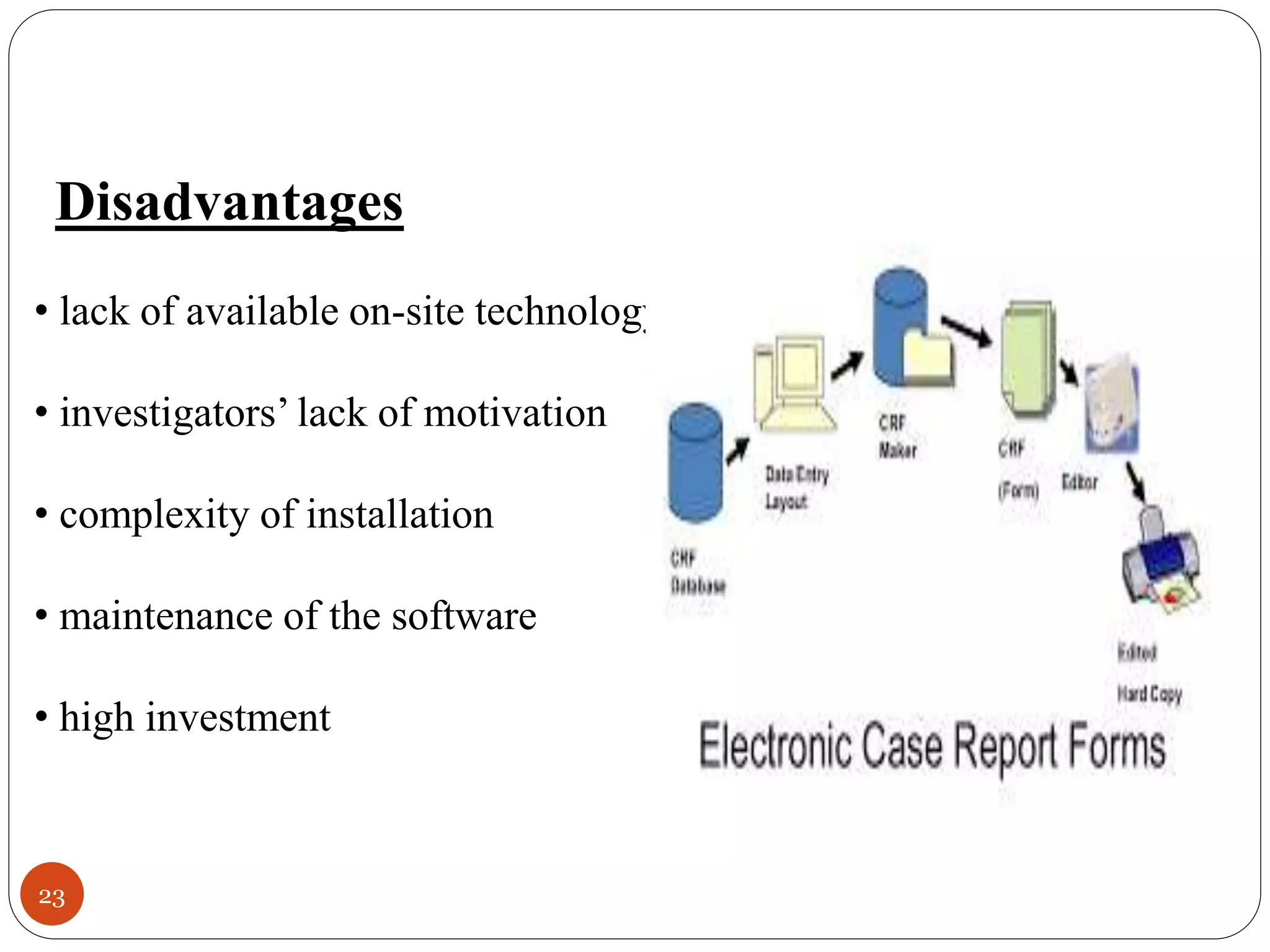
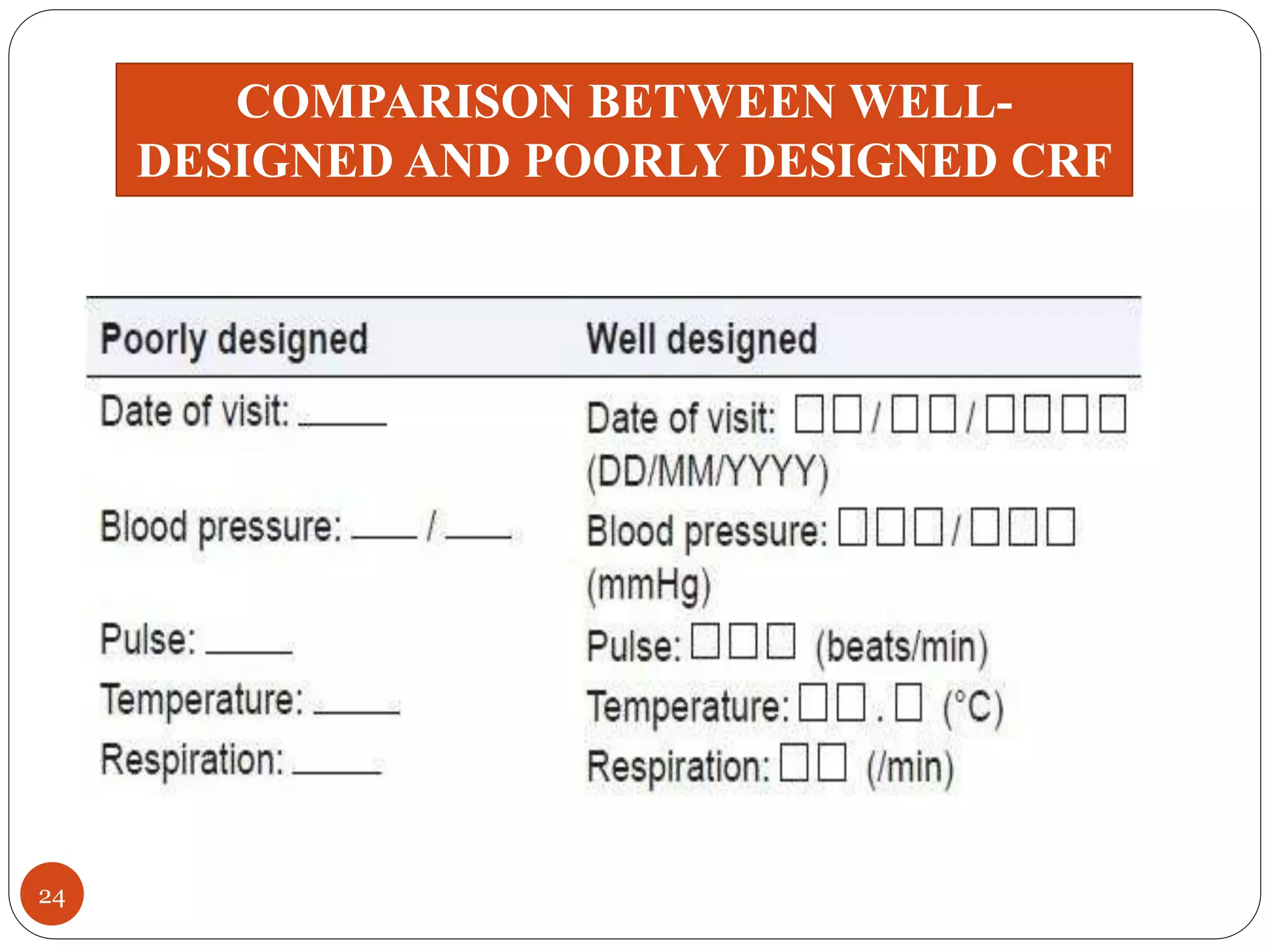
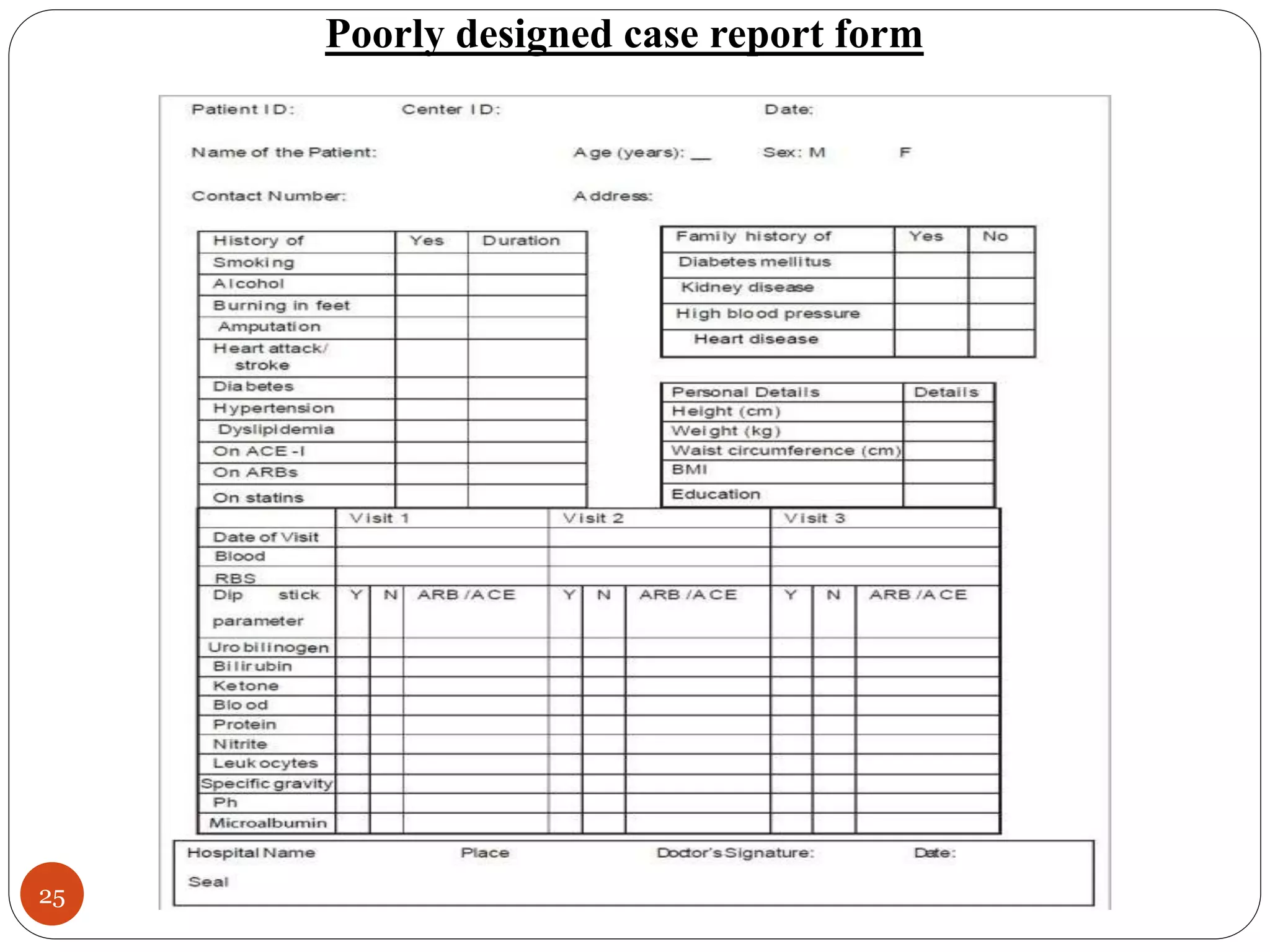
The document discusses case report forms (CRFs), which are used in clinical trials to record patient data. It defines CRFs and explains that they contain all protocol-required information including adverse events. The goals of CRFs are to collect verifiable data according to Good Clinical Practice standards. CRFs can be paper-based or electronic. Well-designed CRFs are structured and formatted consistently to facilitate accurate data collection while avoiding duplication. CRFs provide essential standardized data that is analyzed to advance medical research.












![General Instructions
30
These forms are printed on 3‐part NCR paper.
Please ensure that the guardboard/wrap‐around cover is
inserted between each page before writing.
Press firmly when writing.
Complete the CRF using a black ballpoint pen.
Other colours [pen tips] may not show on all copies.
Ensure that all entries are printed and legible.
Ensure that the header information (i.e. centre no.,
subject’s initials and subject’s ID) is completed consistently
throughout the CRF. If a subject prematurely discontinues
from the trial, the header information and CRF pages must
be completed and a single line drawn across each page.
Ensure that all fields are completed on each page or an
explanation for missing data is recorded on the Comments
page.](https://image.slidesharecdn.com/casereportformandapplication-210912151858/75/Case-report-form-and-application-30-2048.jpg)

