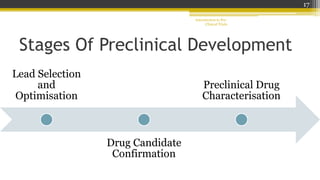
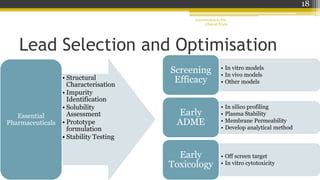
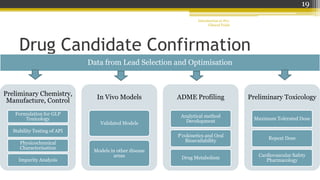
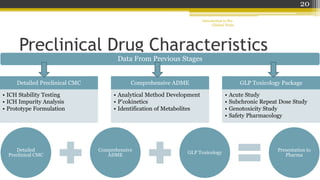
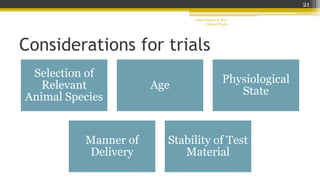
Pre-clinical trials involve testing new drugs, procedures, or medical treatments in animals before beginning clinical trials in humans. They aim to determine safety and efficacy. The document outlines the stages of pre-clinical trials including in vitro and in vivo testing, pharmacokinetic studies, toxicity tests, and FDA review requirements. The goals are to identify safe starting doses in humans, target organs for toxicity, and safety parameters for clinical monitoring before human trials.