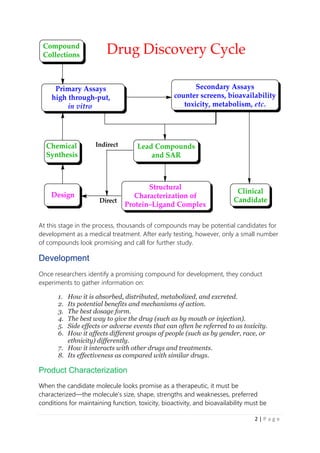
The document summarizes the stages of drug development from discovery through clinical trials and regulatory approval. It describes 10 main stages: 1) discovery and development, 2) preclinical research, 3) investigational new drug application, 4) clinical research including 3 phases of trials, 5) FDA review and approval, and 6) post-market safety monitoring. Preclinical research involves testing for safety and efficacy in animal and lab models. If promising, the drug enters clinical trials with humans starting with small Phase 1 safety studies, then Phase 2 dosing studies, and larger Phase 3 trials to confirm efficacy before the FDA reviews the final application for approval. The overall process takes around 10-15 years from discovery to patients.