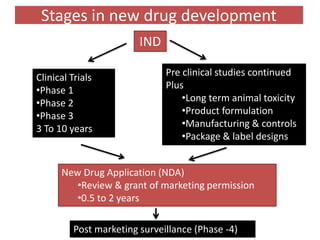
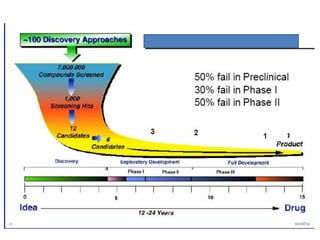
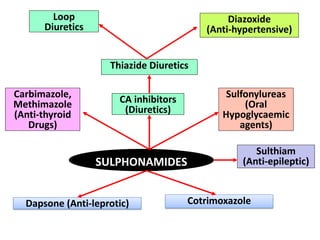
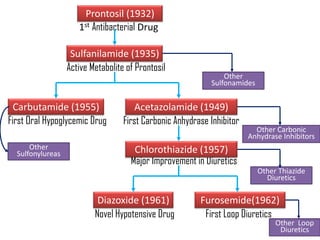
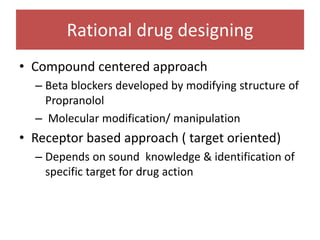
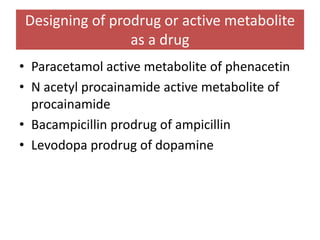
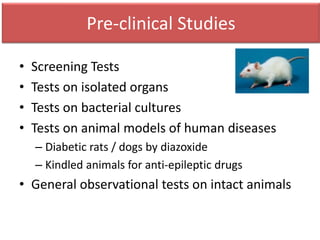
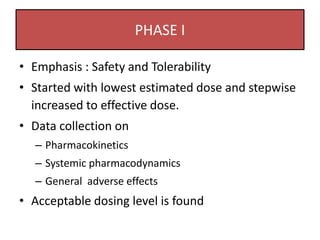
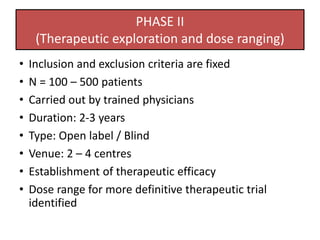
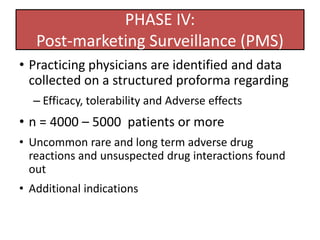
The document outlines the stages of new drug development, beginning with the drug discovery phase that involves synthesis and isolation of chemical entities, followed by preclinical studies, and clinical trials through several phases, culminating in marketing approval and post-marketing surveillance. It discusses various approaches to drug discovery including random screening, serendipity, and rational drug design, while emphasizing the importance of toxicological and pharmacokinetic studies during preclinical testing. Additionally, regulatory guidelines for good clinical practice (GCP) are highlighted, along with the phases of clinical trials that assess drug safety and efficacy.