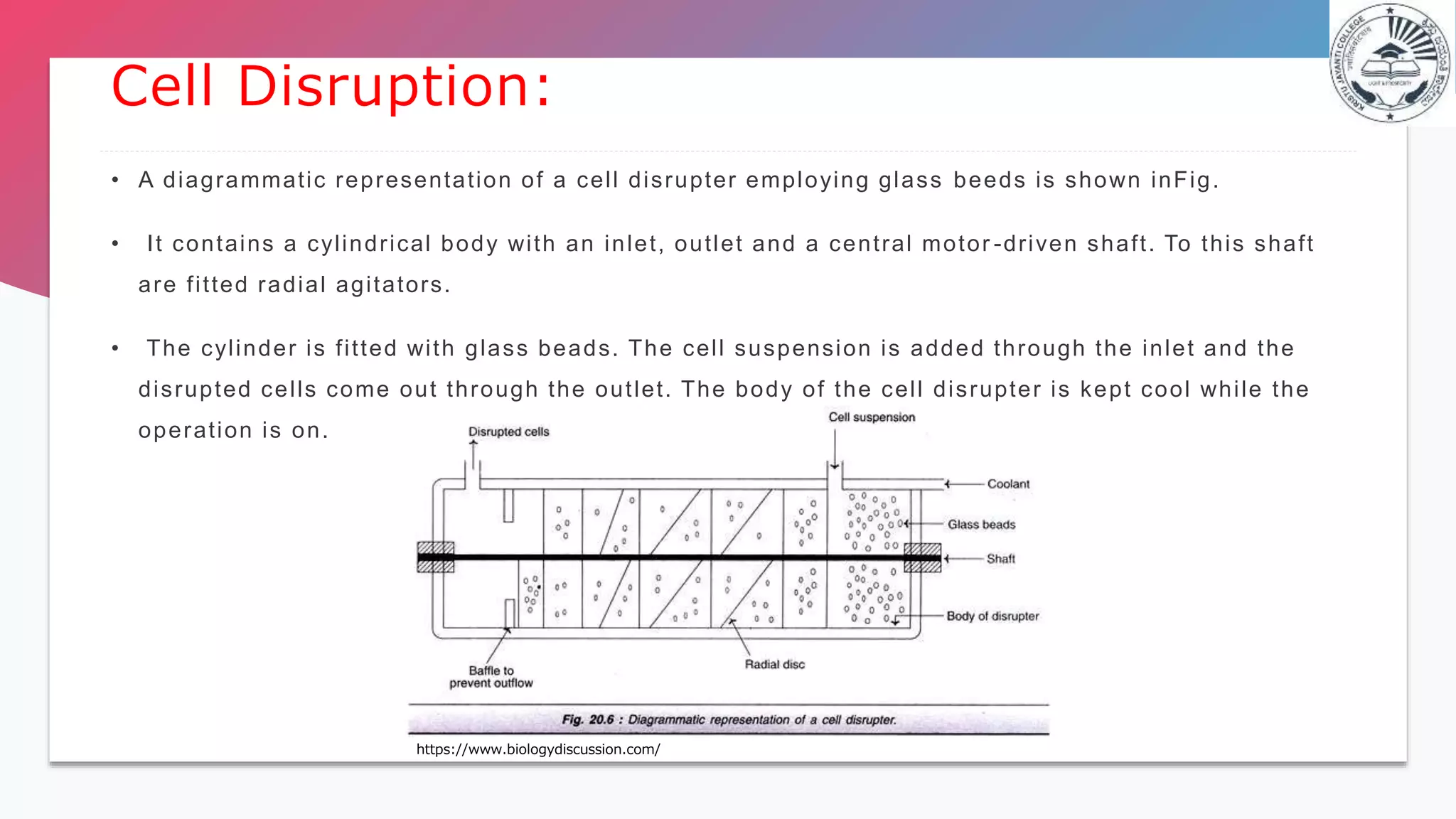
This document discusses various methods for releasing intracellular products from cells, including physical, chemical, and enzymatic methods. Physical methods include ultrasonication, osmotic shock, heat shock, high pressure homogenization, impingement, and grinding with glass beads. Chemical methods involve using alkalis, organic solvents, and detergents. Enzymatic methods commonly use lysozyme. After release, concentration techniques such as evaporation, liquid-liquid extraction, membrane filtration, precipitation, and adsorption are used to remove water and achieve product concentration. Chromatography is also discussed as an effective purification technique.