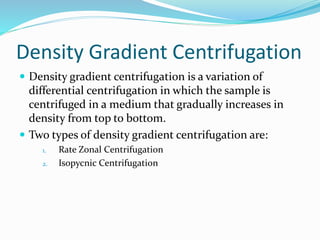
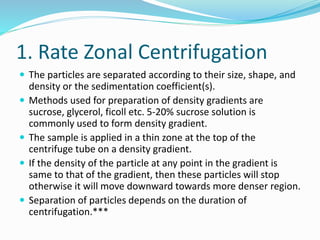
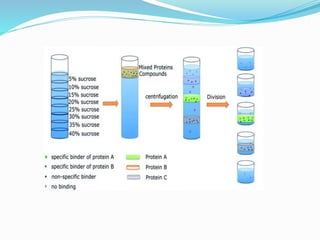
This document discusses subcellular fractionation, which is the process of separating intact organelles from homogenized cells and tissues using differential centrifugation. It begins by introducing the cell and its organelles. It then covers the history of the technique, methods for homogenizing cells, and the two main centrifugation methods - differential and density gradient centrifugation. Marker enzymes are also discussed as a way to identify isolated organelles. The summary provides an overview of the multi-step centrifugation process and identifies marker enzymes for different organelle fractions.









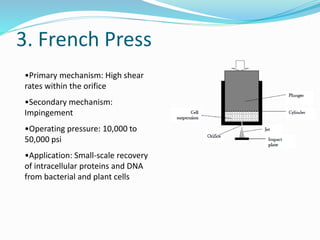


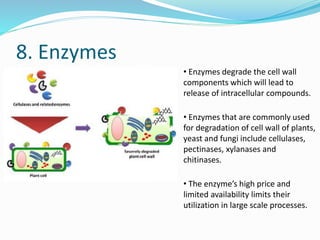




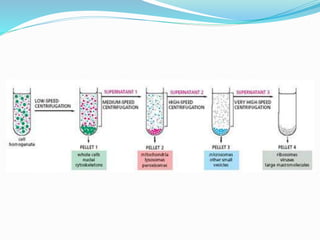


![Summary
Homogenate
[in isotonic buffer]
(Centrifuge at
2000*g for 10 min)
Pellet
[Nuclei]
(Marker Enzyme – DNA
Polymerase)
Supernatent
(Centrifuge at 10,000*g
for 20 min)
Pellet
[Mitochondria]
(Marker - Succinate
dehydrogenase and
Monoamine oxidase)
Supernatent
[Post Mitochondrial
Supernatent]
(Centrifuge at
1,05,000*g for 4 hrs)
Pellet
[Microsome]
(Plasma membrane, ER,
Golgi, Lysosome,
Peroxisome)
Supernatent
[Cytosol containing
ribosomes and other
macromolecules]](https://image.slidesharecdn.com/subcellularfractionationandmarkerproteins-190408201552/85/Subcellular-fractionation-and-marker-proteins-38-320.jpg)

