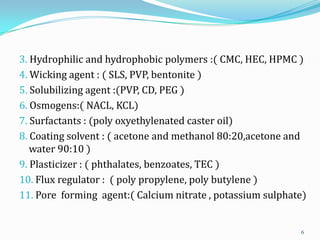
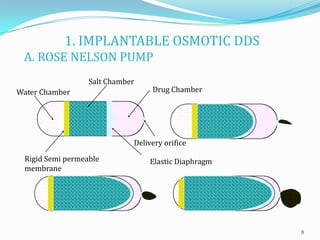
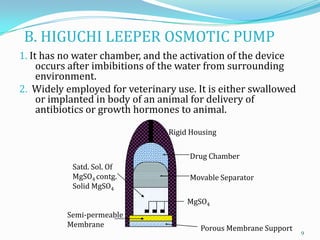
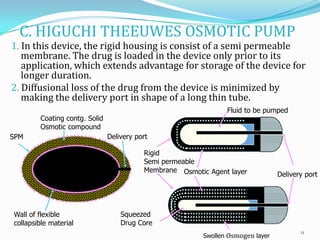
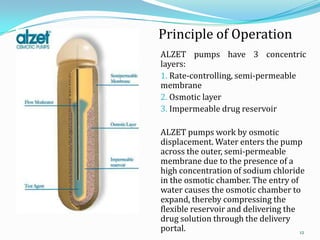
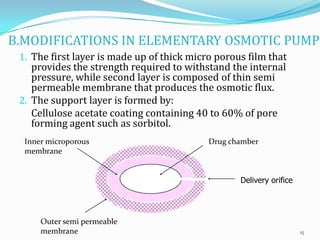
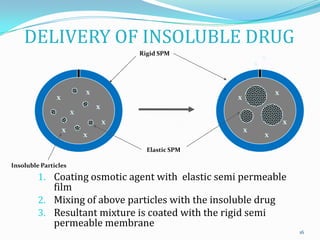
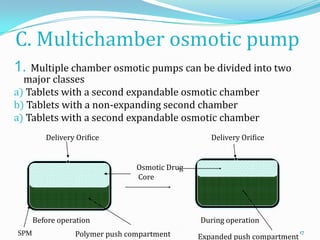
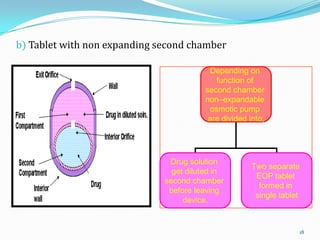
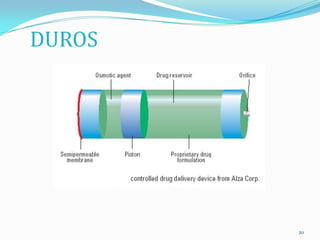
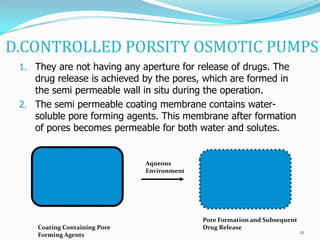
The document discusses osmotic drug delivery systems. It defines osmosis and osmotic pressure, and describes the basic components of osmotic drug delivery systems including semipermeable membranes, osmogens, and drug formulations. It classifies osmotic systems as implantable or oral, and describes several types of oral osmotic pumps including elementary, modified, multi-chamber, controlled porosity, and monolithic systems. Key factors that affect drug release are also outlined. The document concludes by listing several marketed osmotic products.