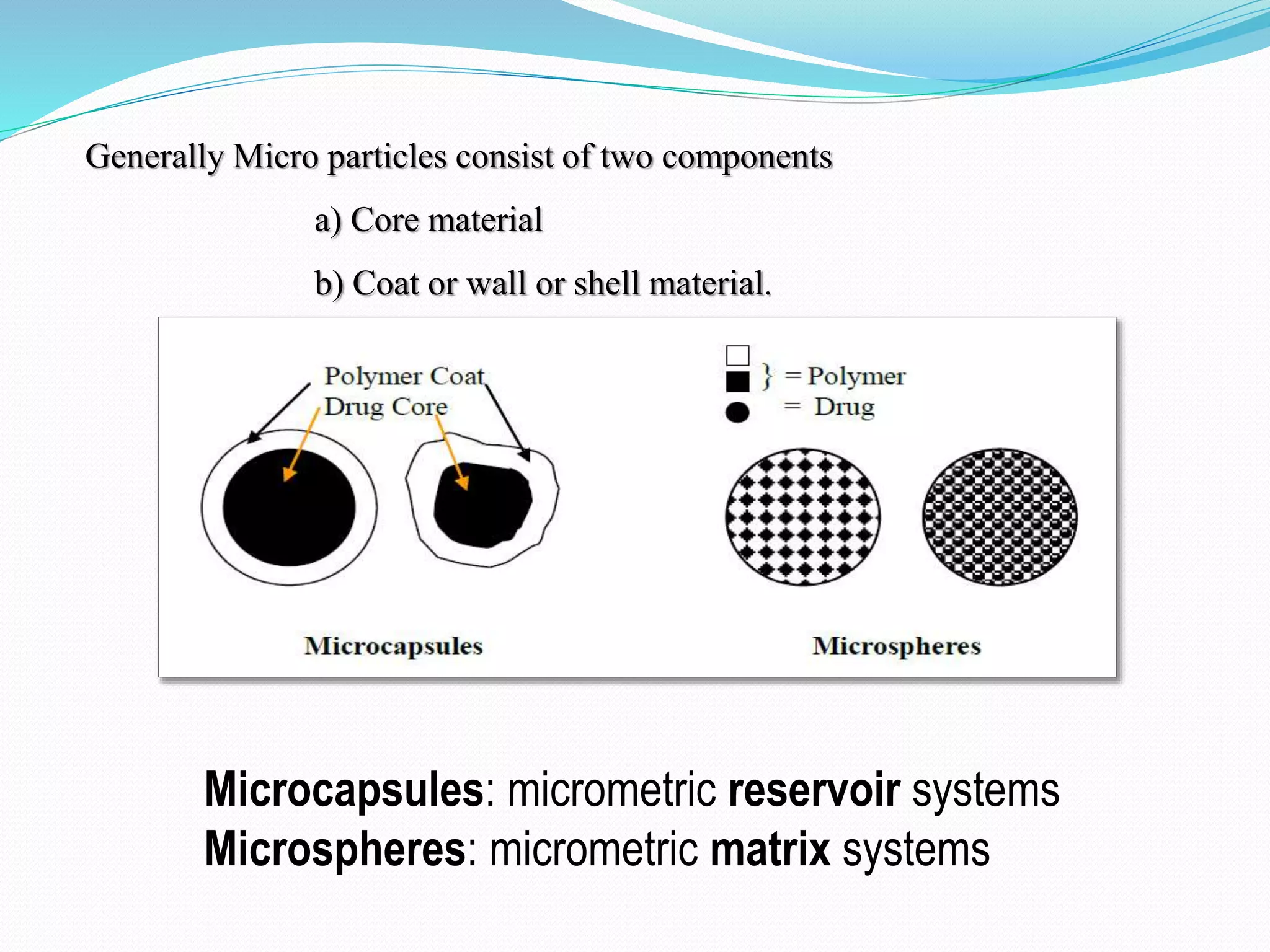
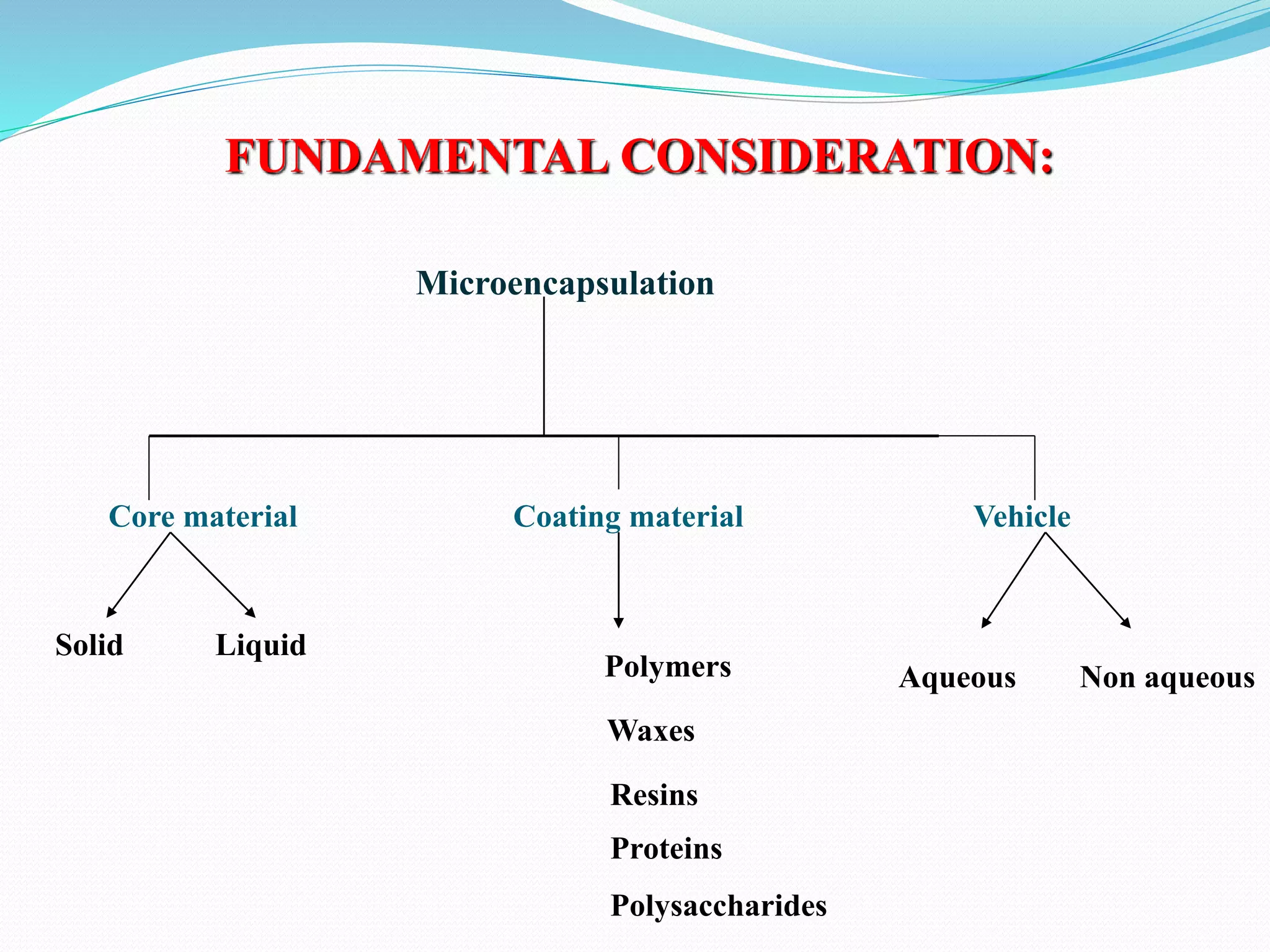
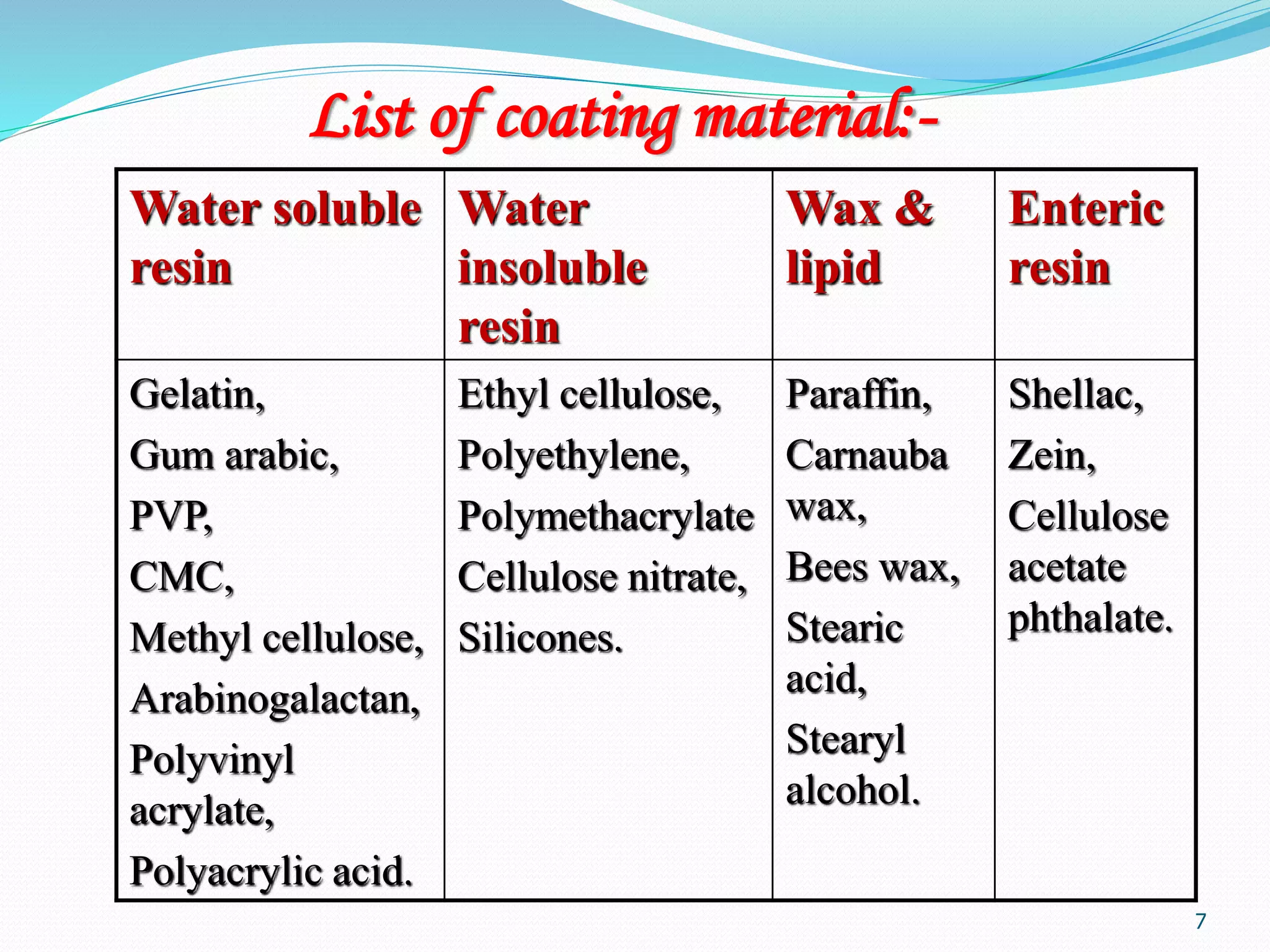
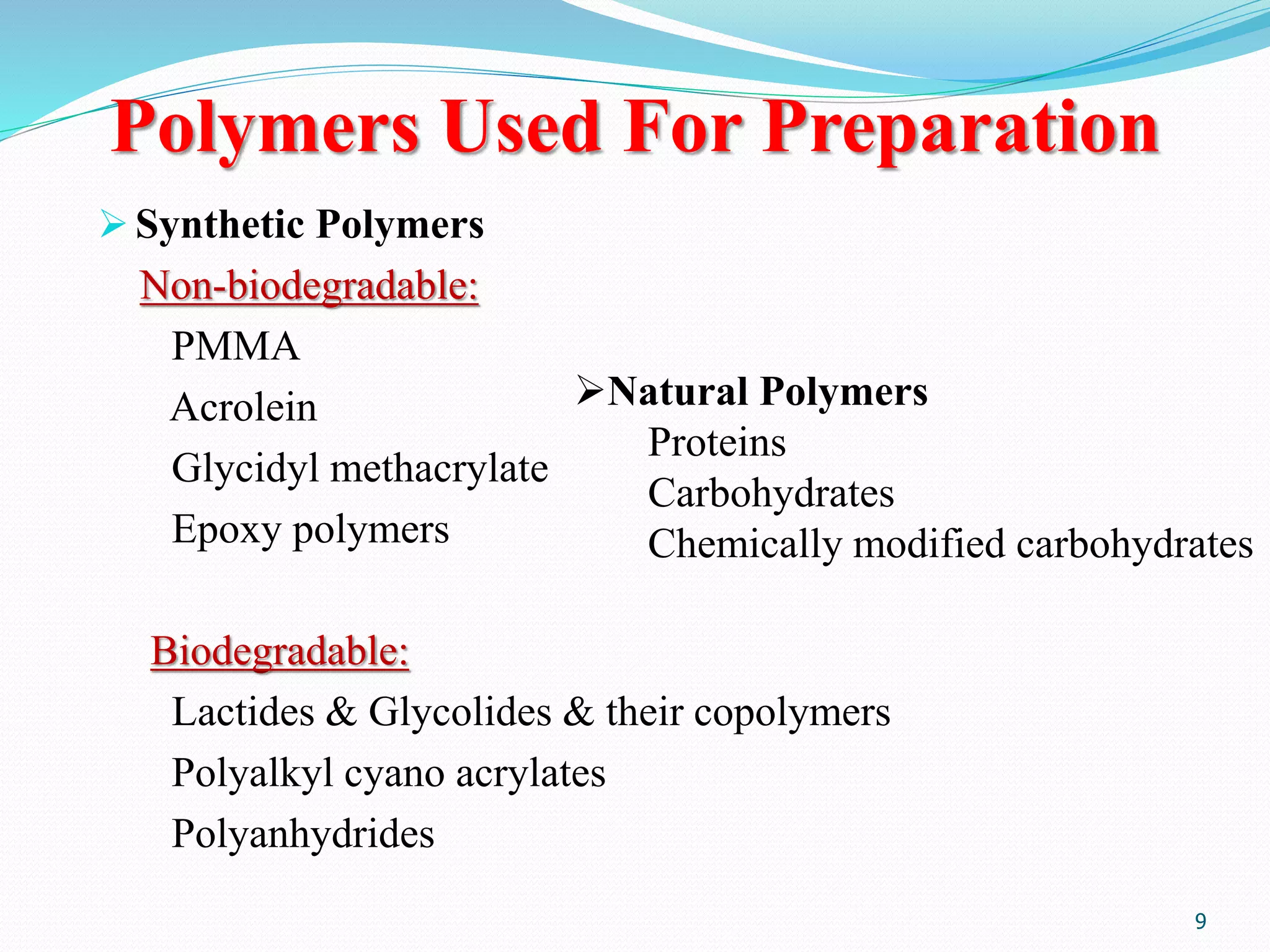
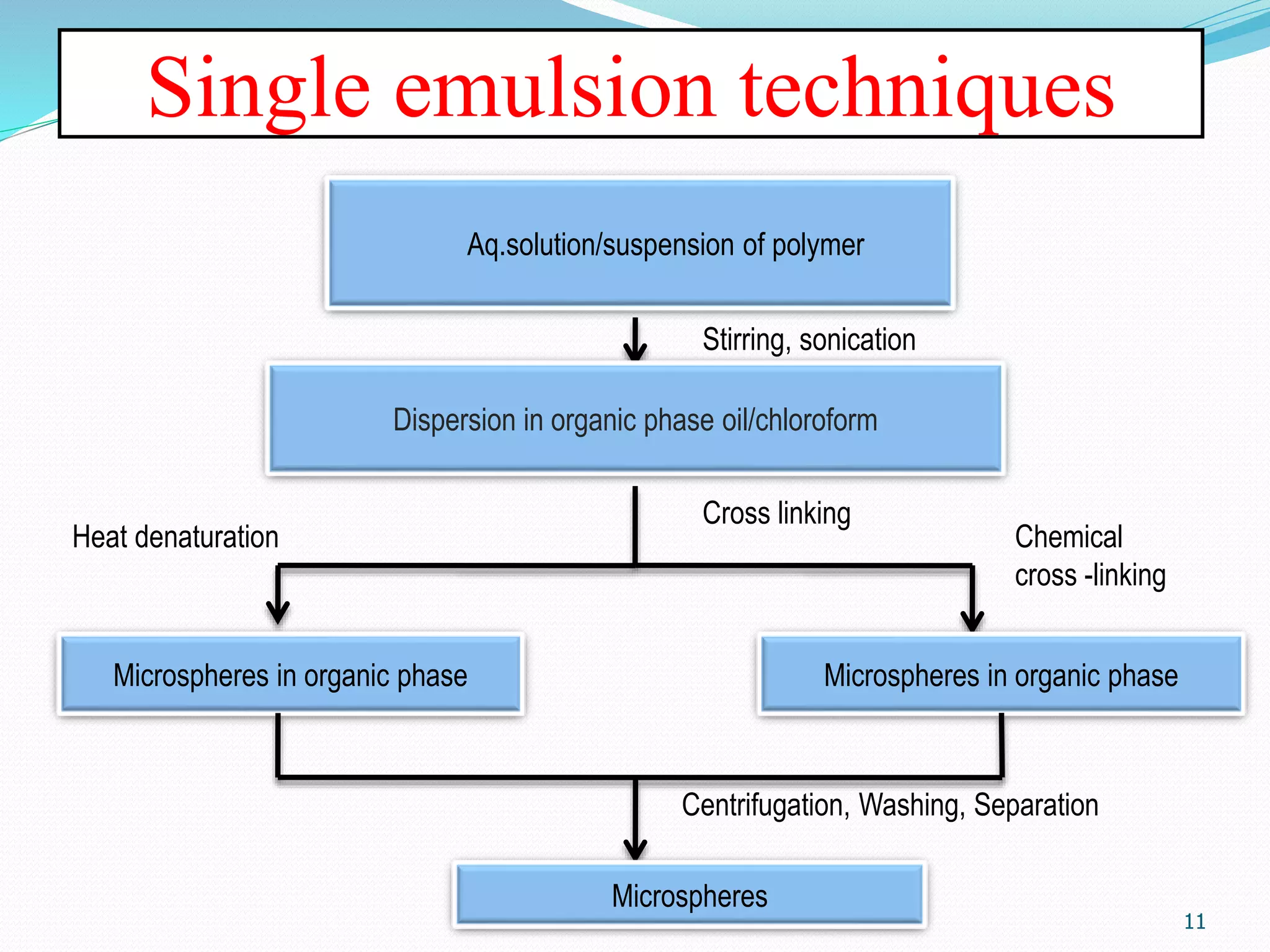
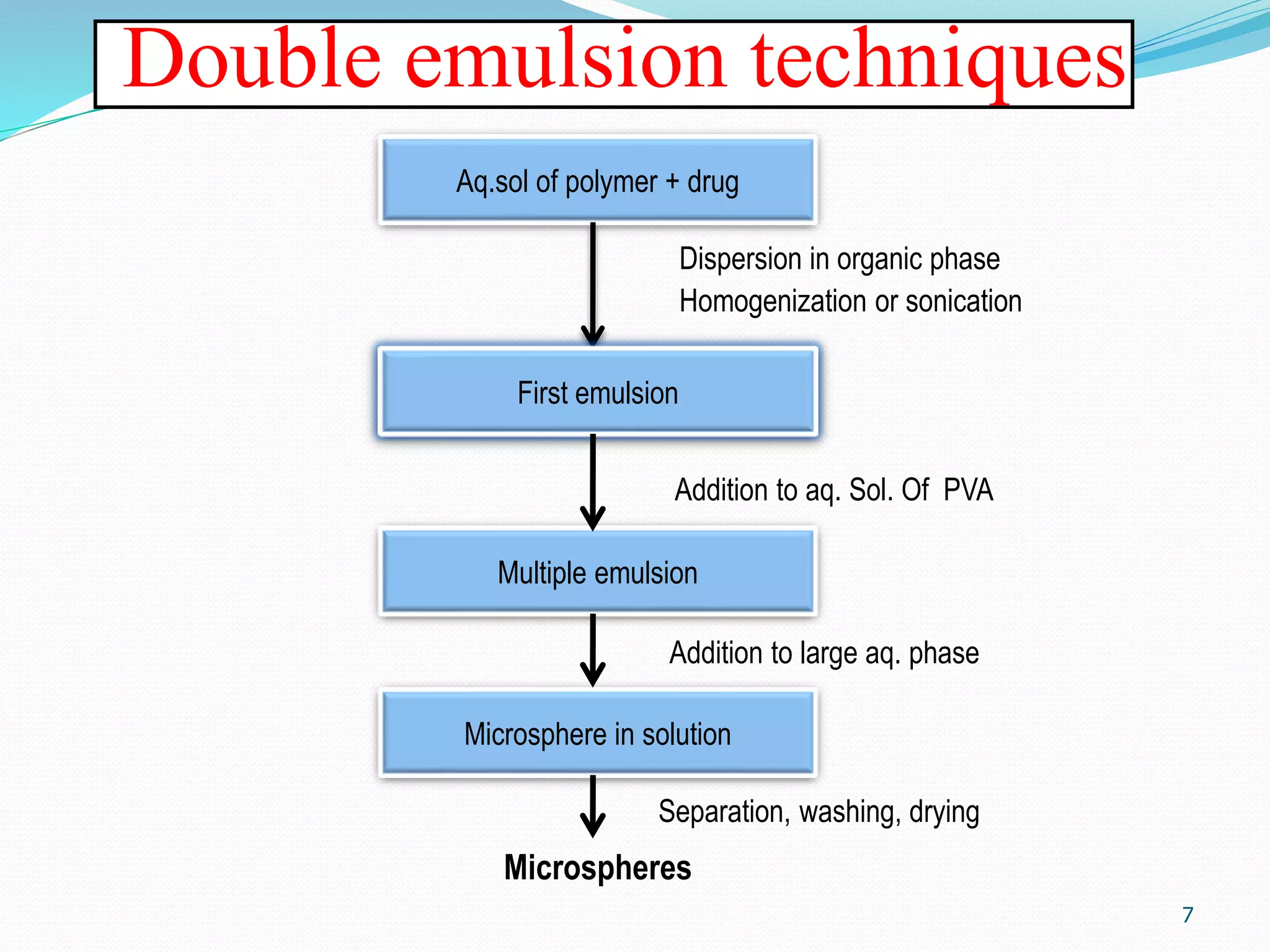
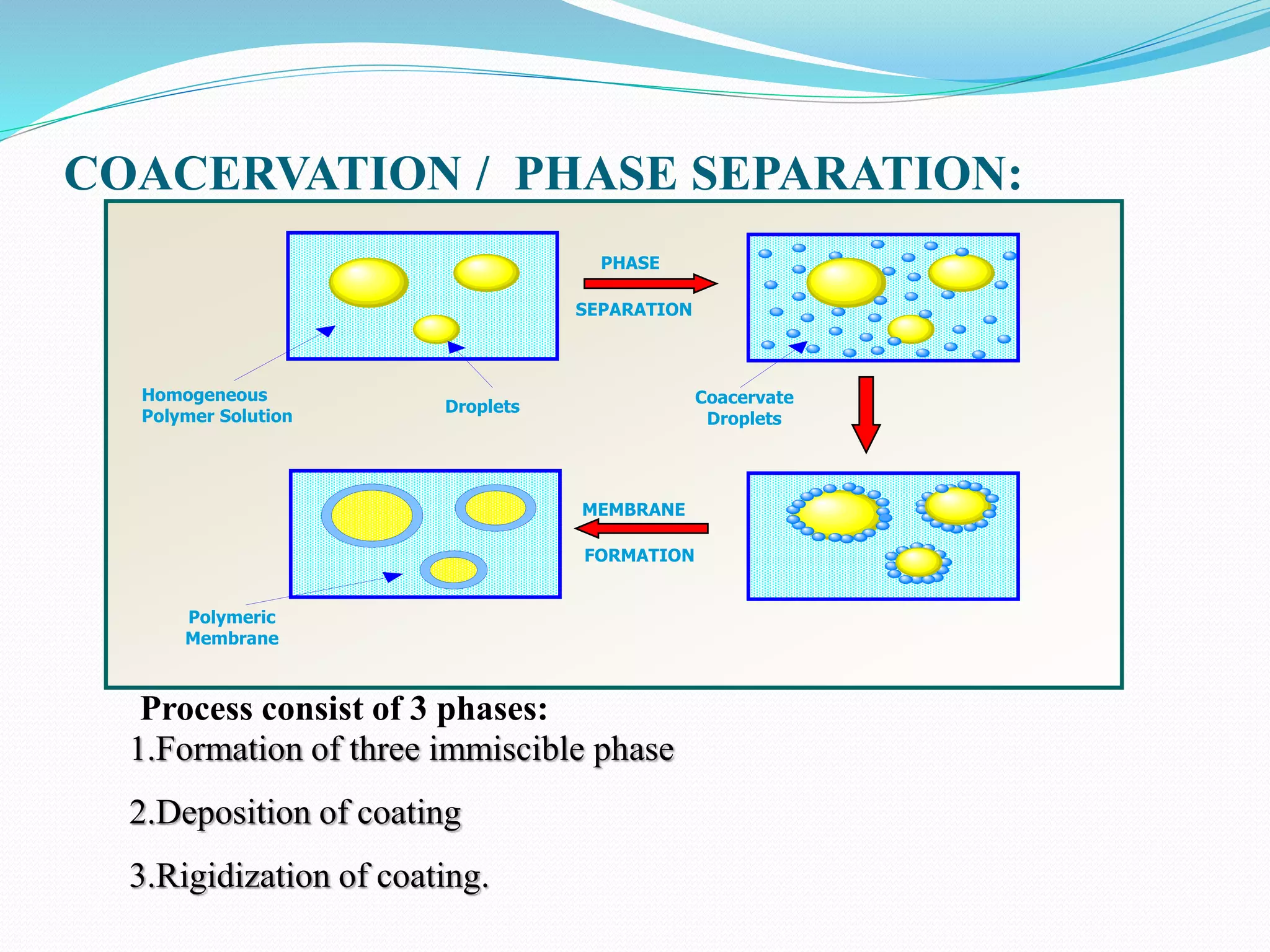
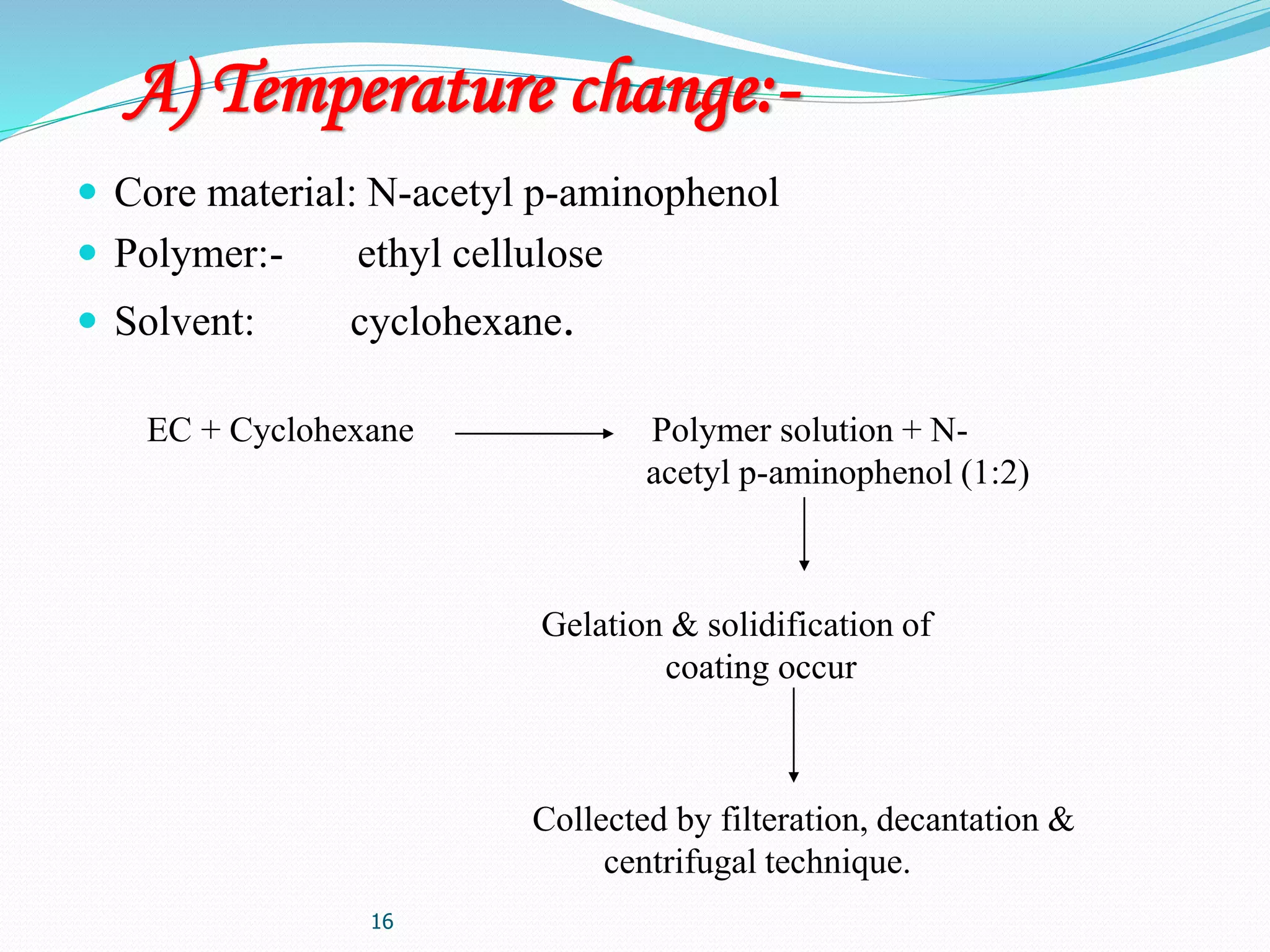
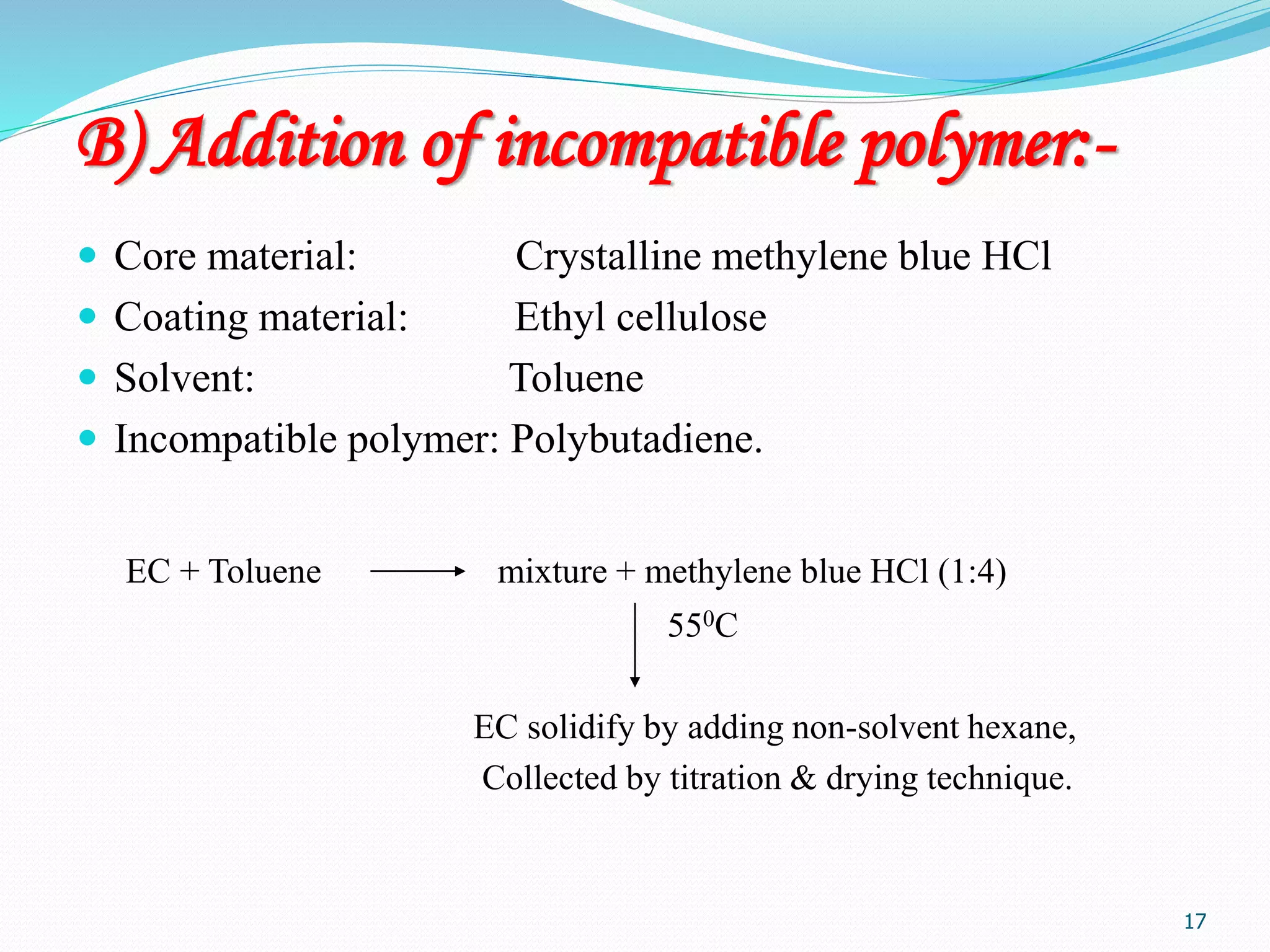
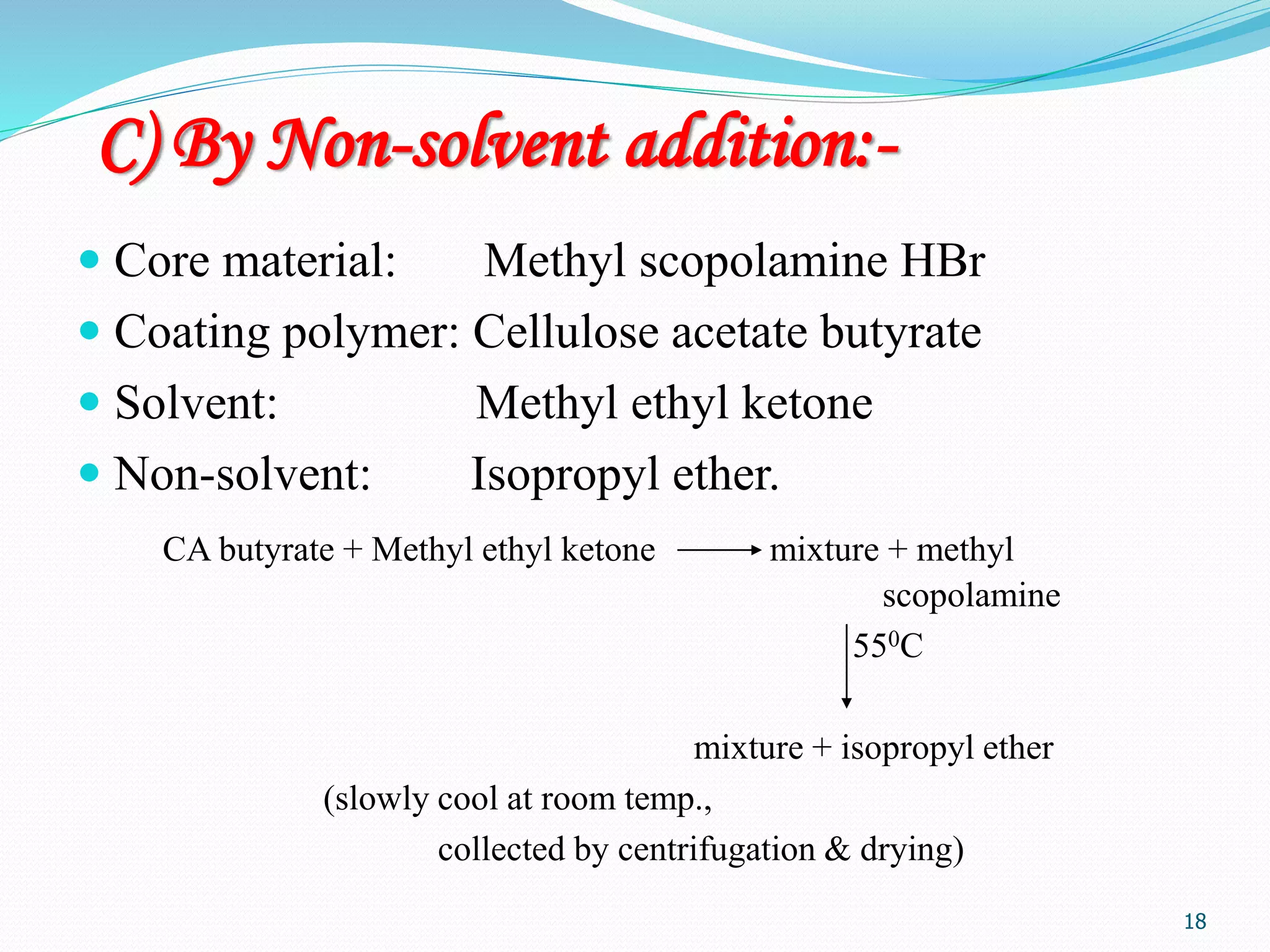
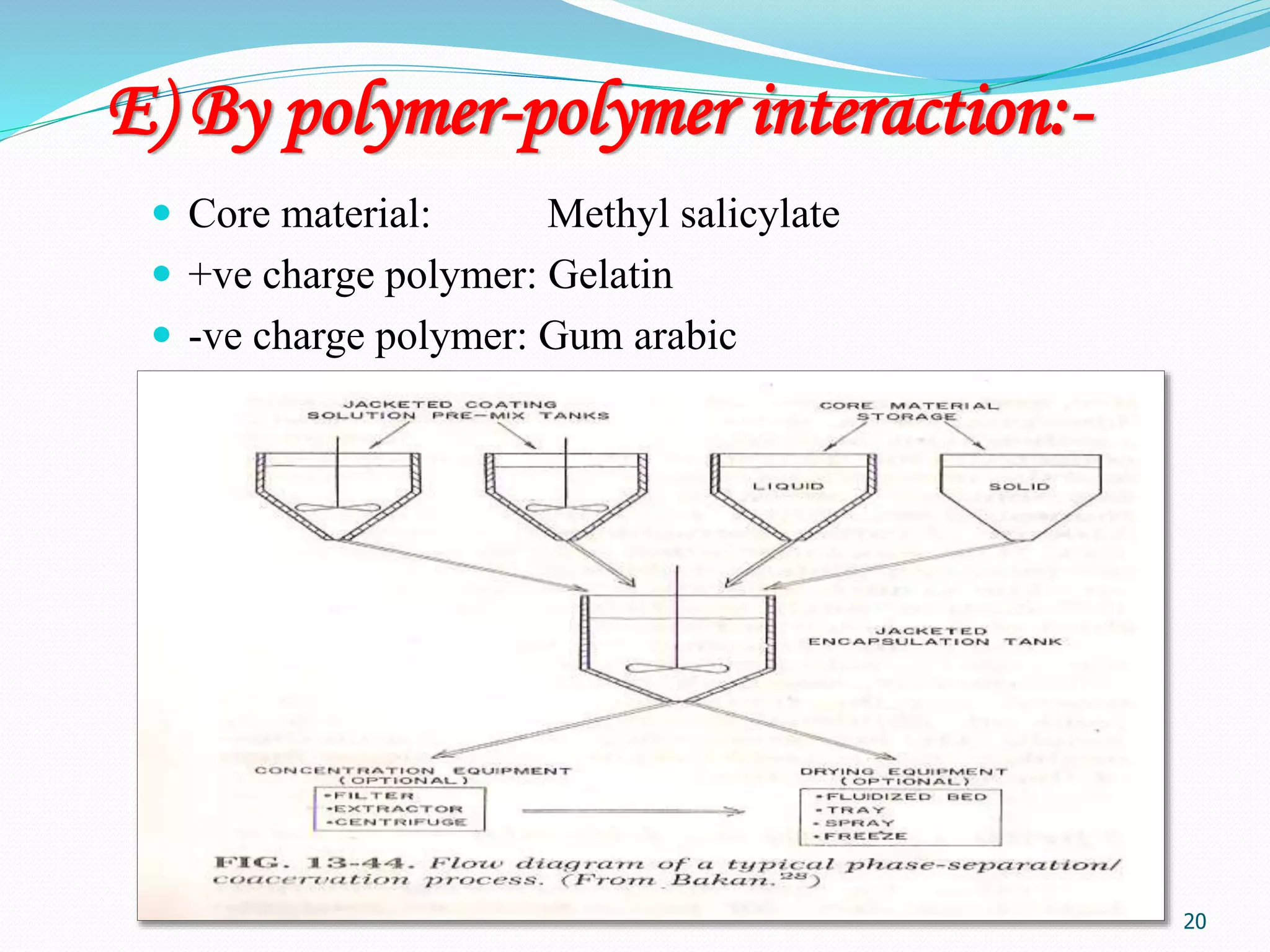
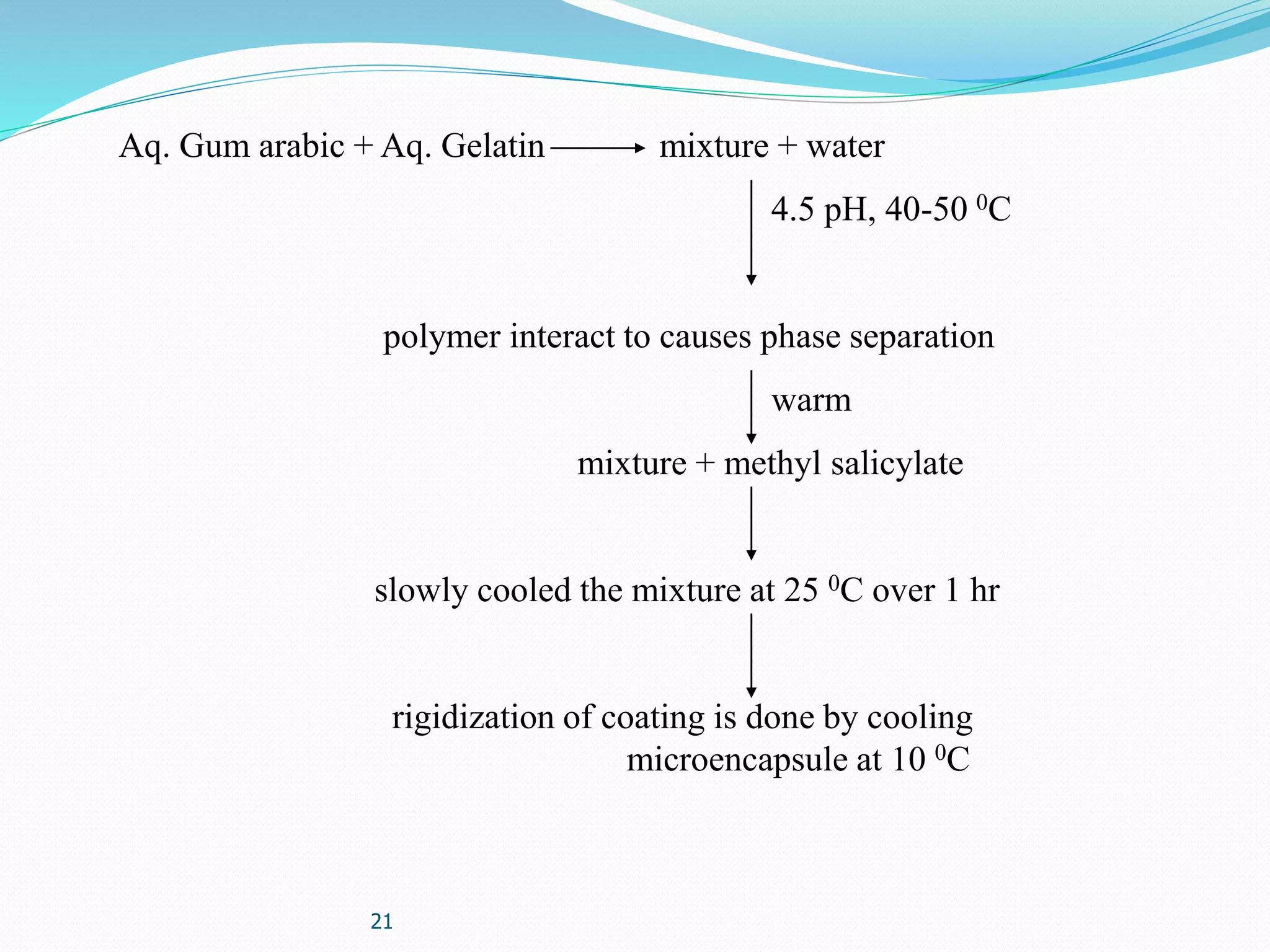
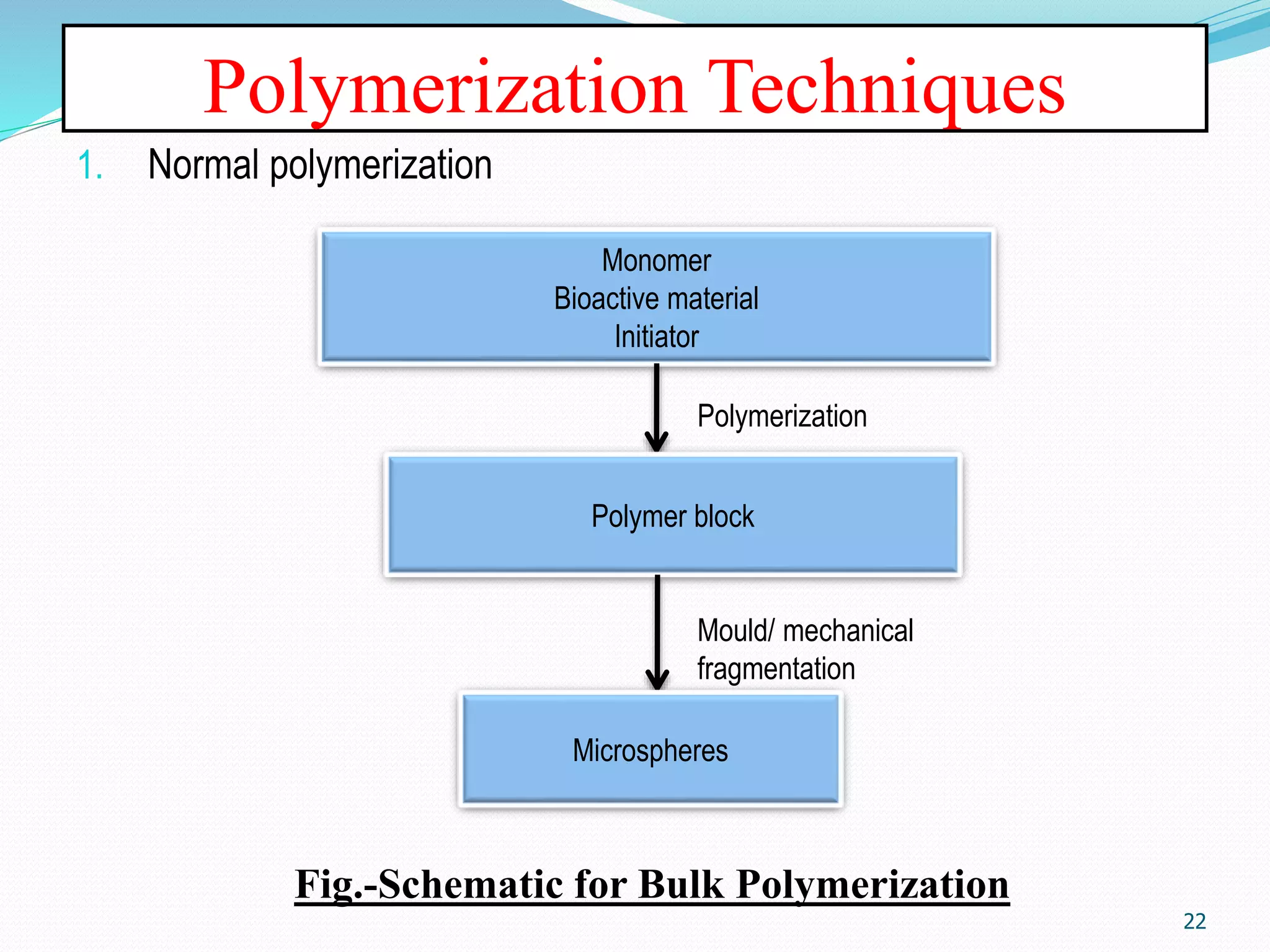
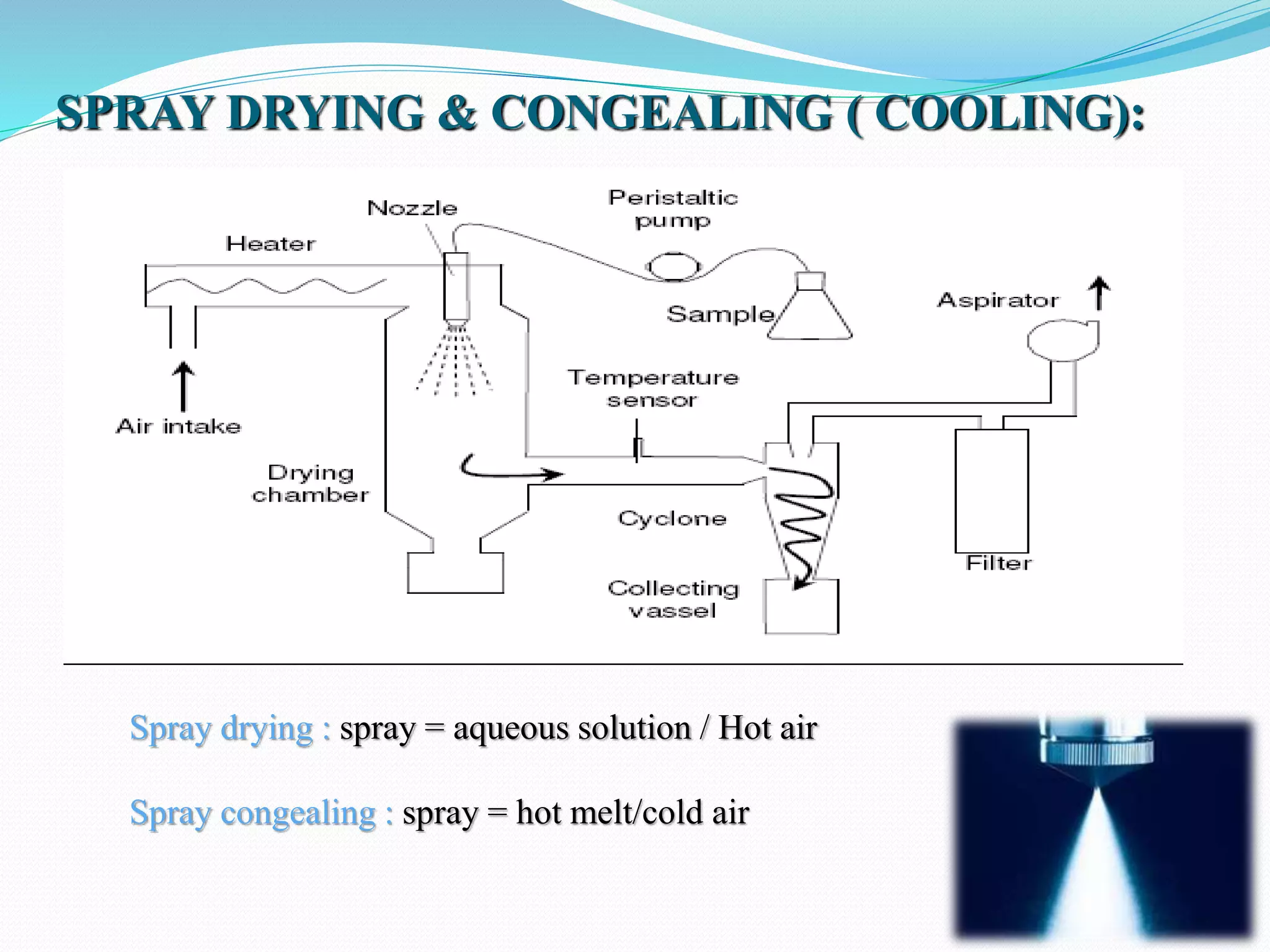
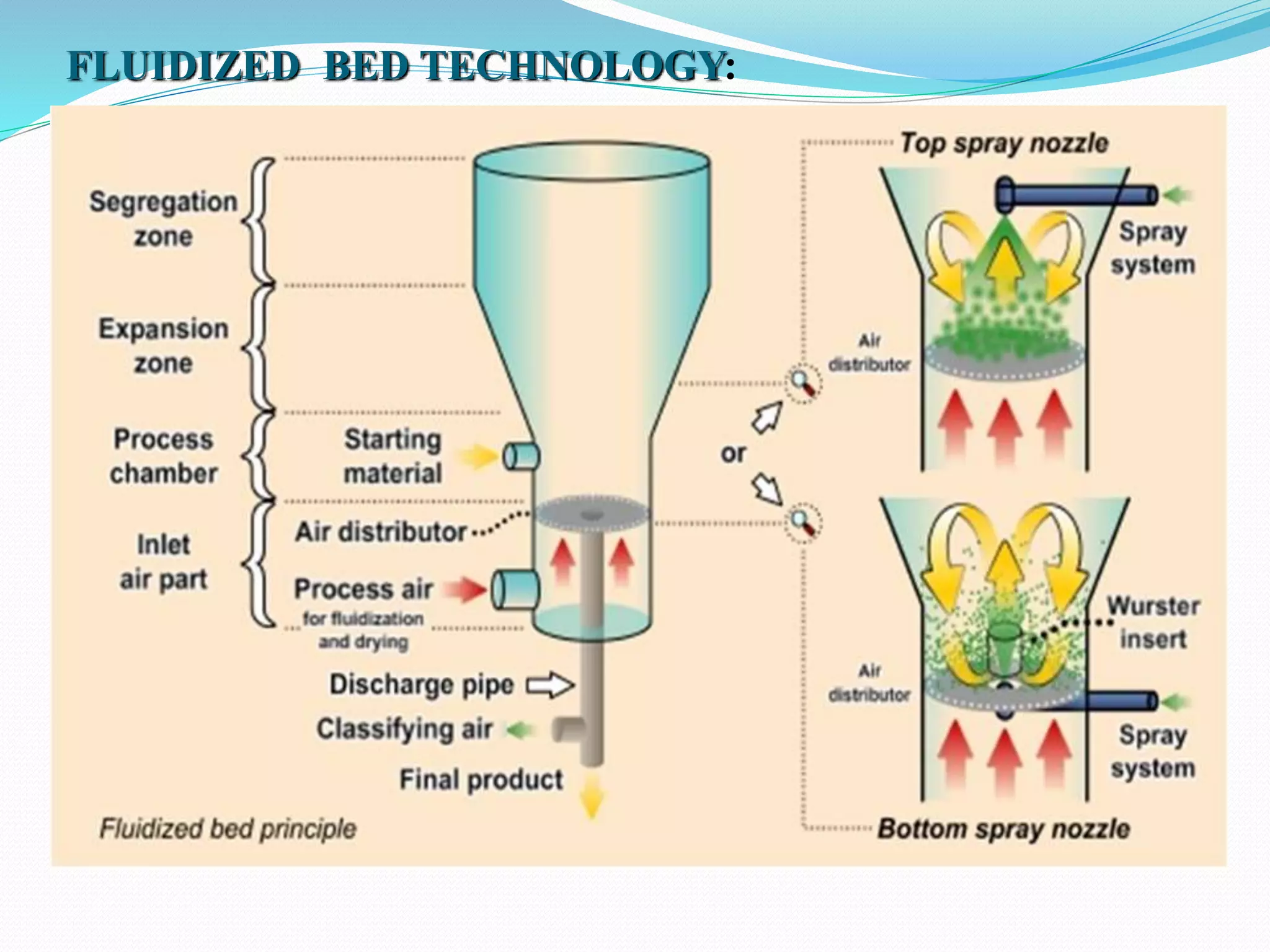
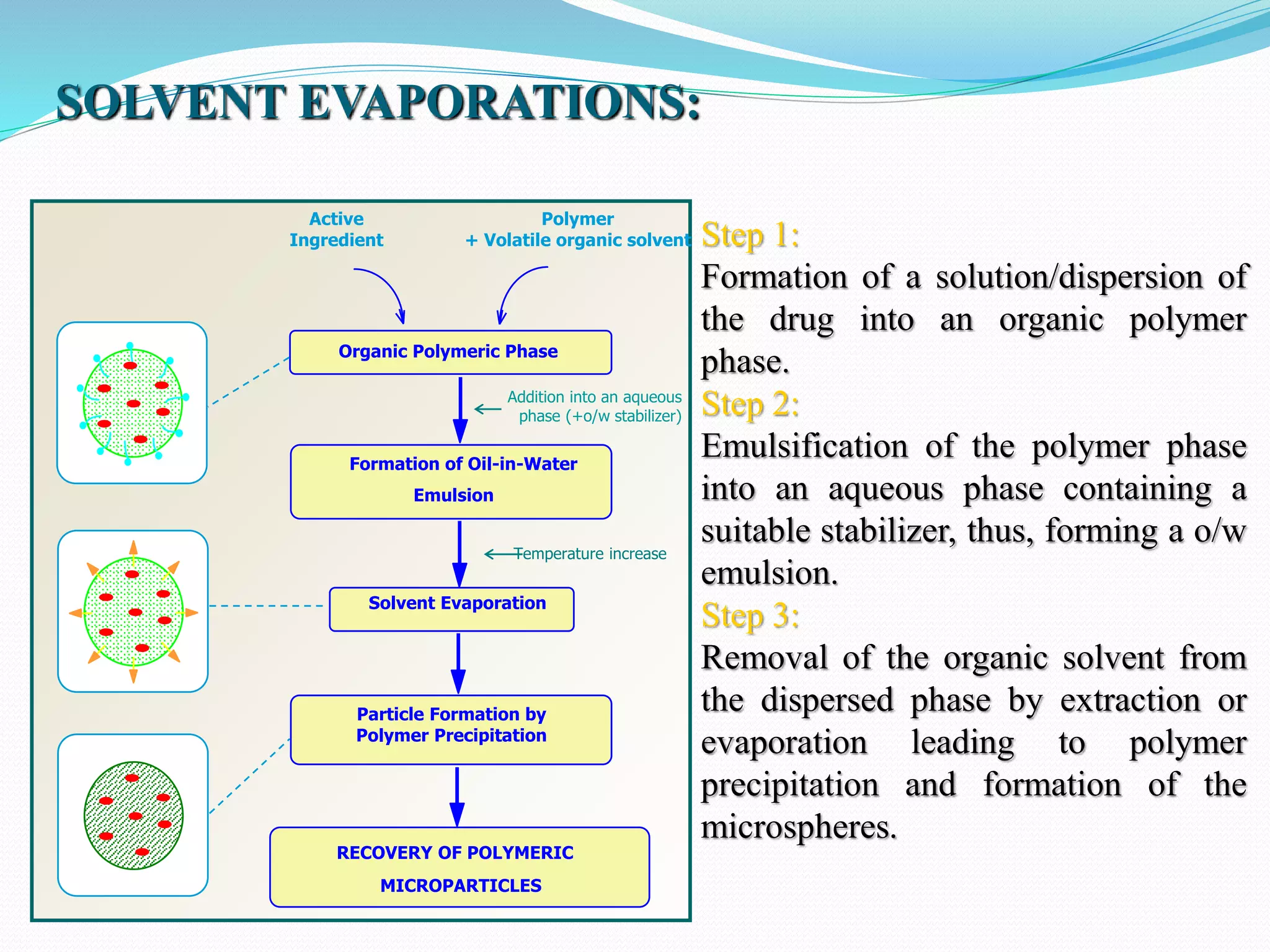
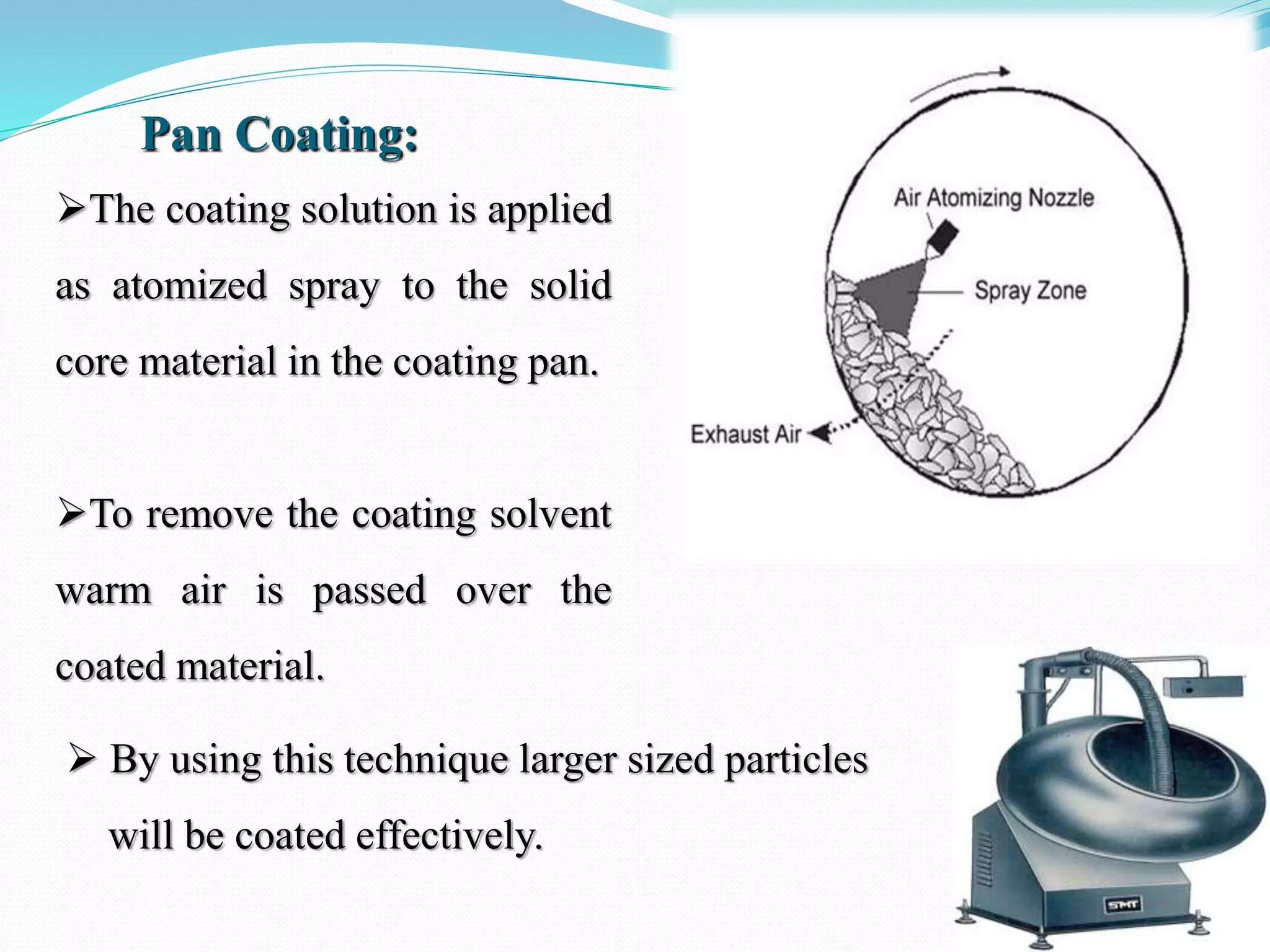
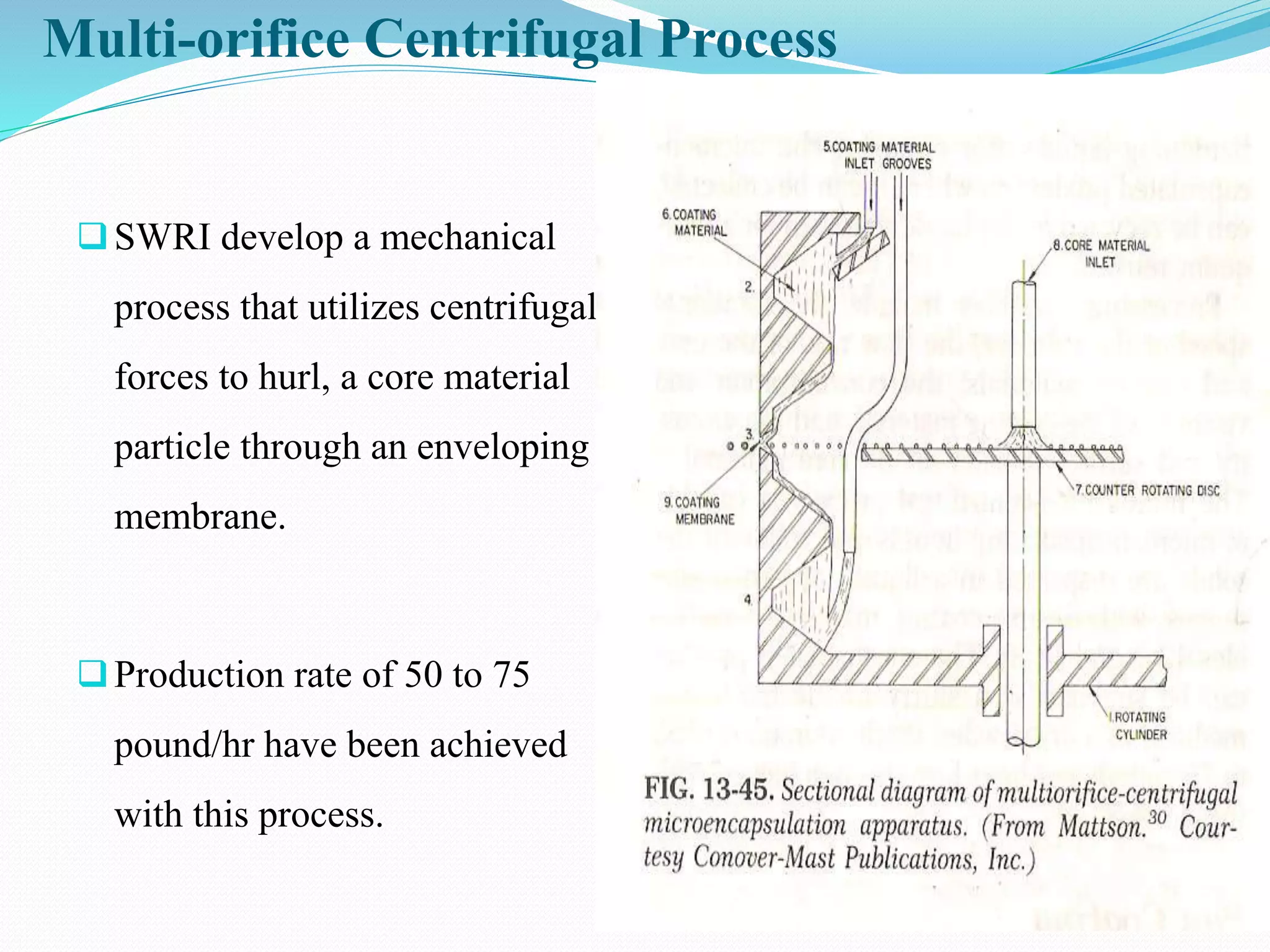
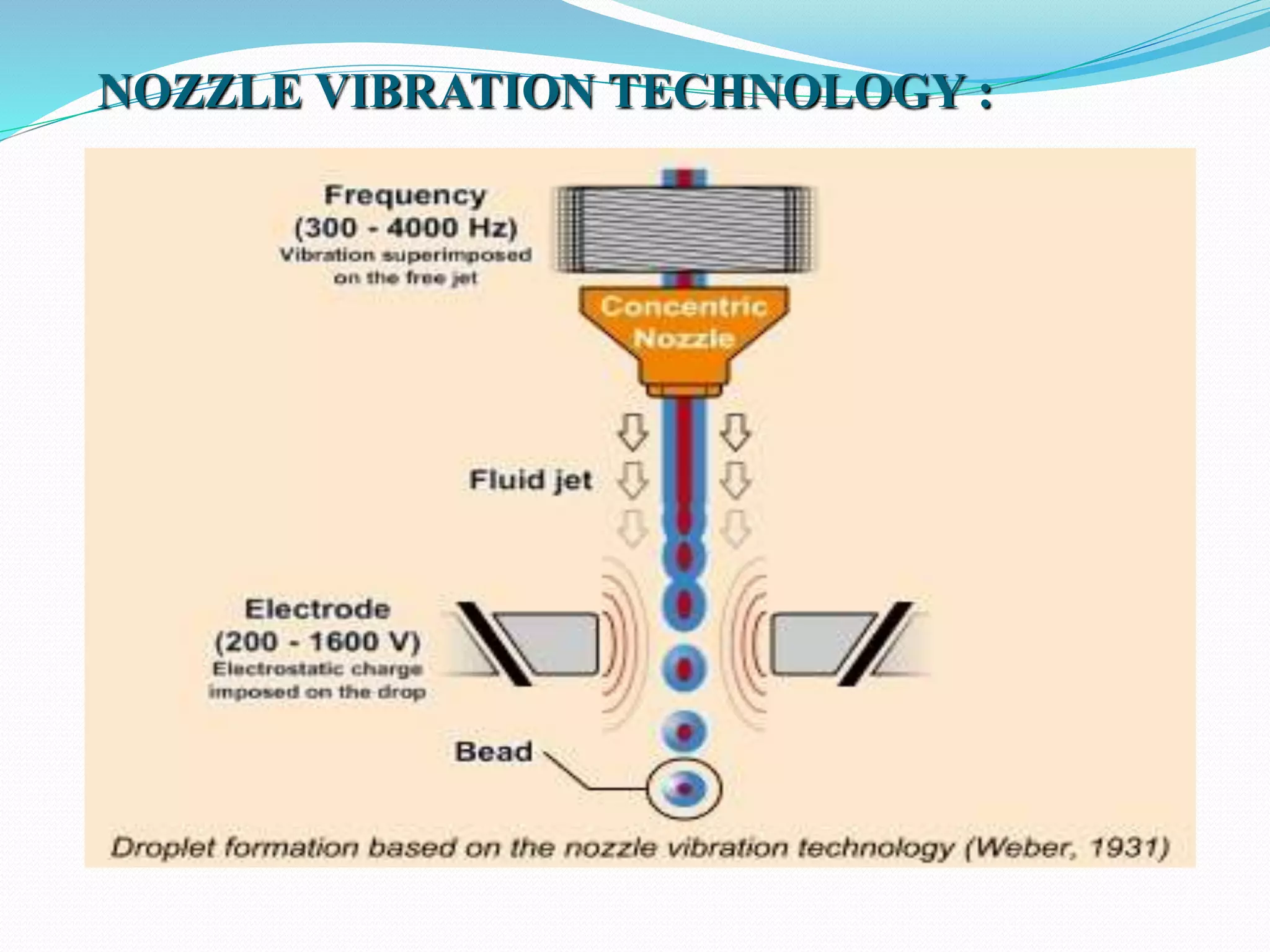
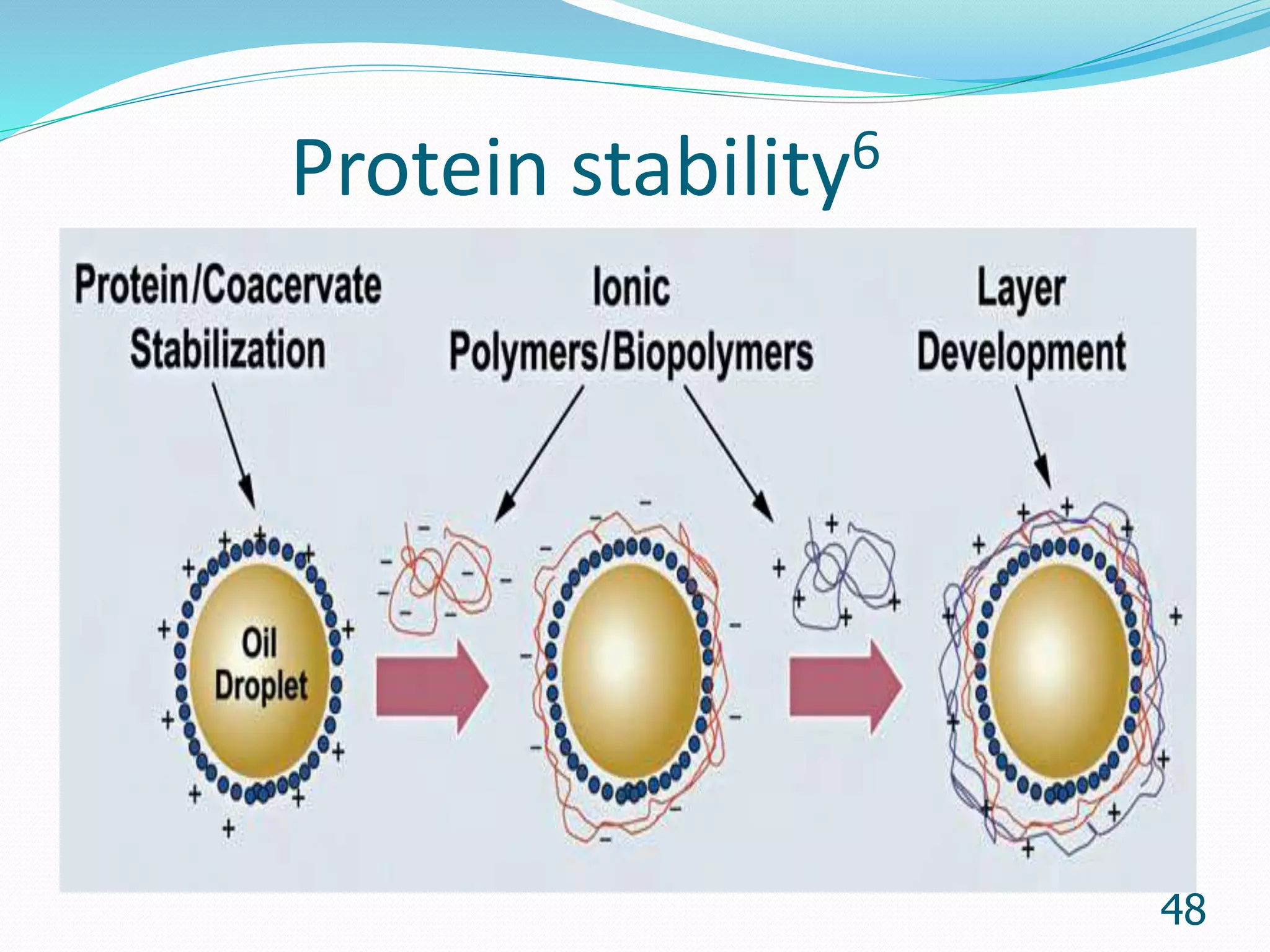
Microencapsulation is a process where core materials are surrounded by a coating to form microparticles or microcapsules between 3-800 μm in size. It can be used to increase bioavailability, alter drug release, improve compliance, enable targeted delivery, and mask tastes. Various techniques like coacervation, spray drying, solvent evaporation, and pan coating can be used. Polymers are common coating materials and microencapsulation can protect core materials, control reactivity, and convert liquids to solids. The microparticles are evaluated based on morphology, drug content, particle size, and dissolution studies.