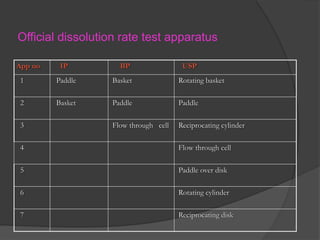
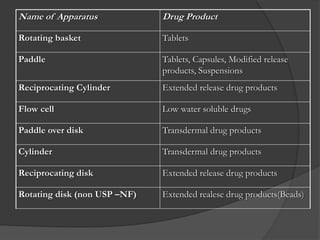
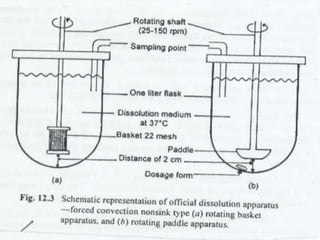
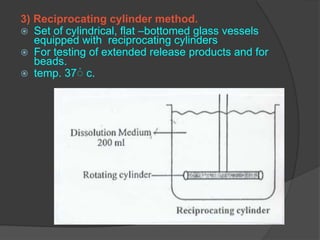
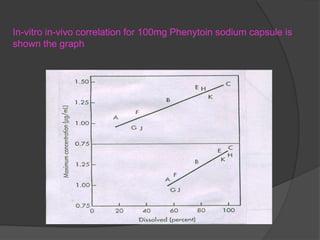
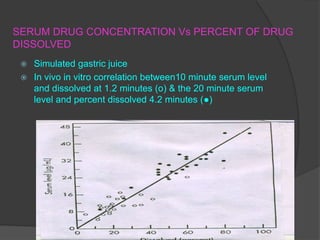
This document summarizes a seminar on in vitro dissolution testing models. It discusses the need for dissolution testing in evaluating bioavailability and ensuring quality. The main official dissolution apparatus described are the rotating basket, paddle, reciprocating cylinder, and flow-through cell methods. In vitro-in vivo correlation seeks to correlate dissolution results with bioavailability. Dissolution acceptance criteria and various non-official dissolution testing methods are also outlined.