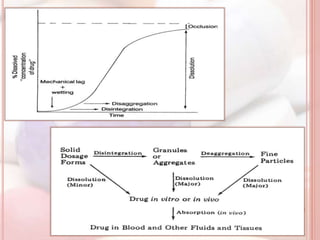
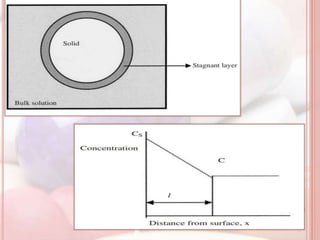
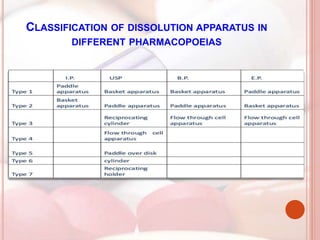
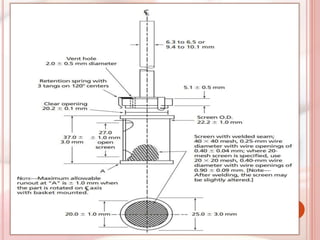
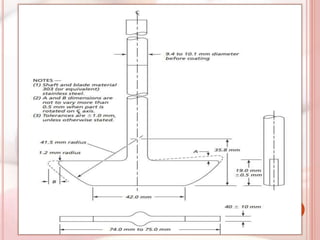
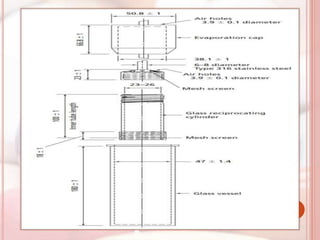
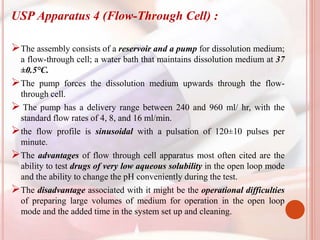
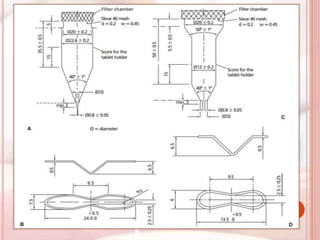
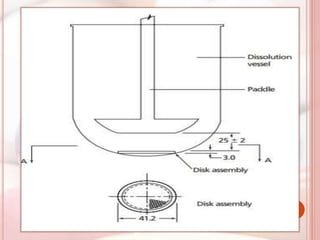
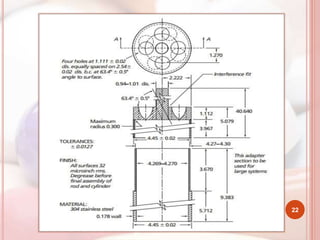
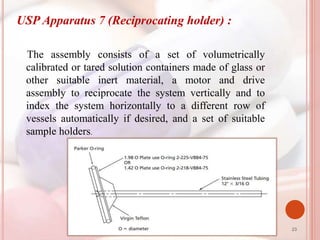
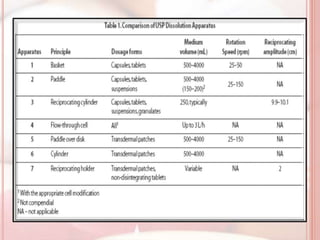
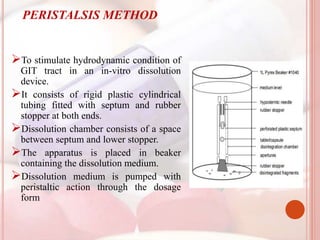
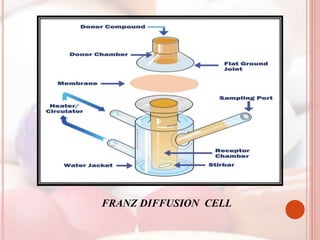
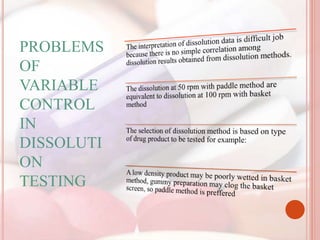
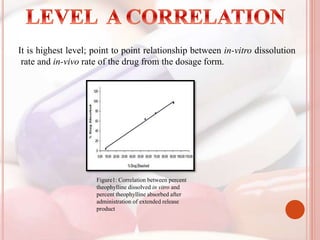
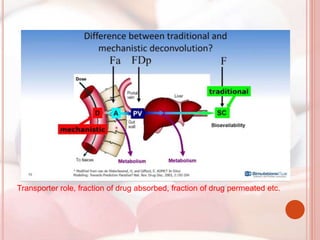
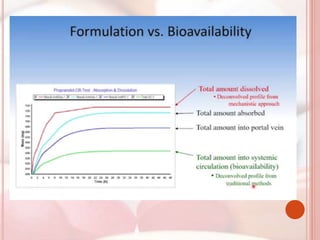
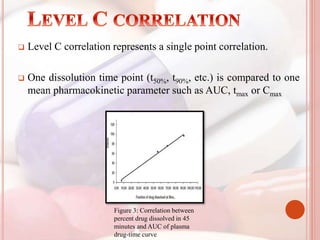
This document discusses in vitro dissolution, which is the process by which a solid substance dissolves in a solvent to form a solution. It describes the various processes involved in dissolution of solid dosage forms and defines intrinsic dissolution rate. It also provides the mathematical equations to describe dissolution processes under sink and non-sink conditions. The document then discusses various compendial dissolution apparatus and methods specified in different pharmacopoeias including rotating basket, paddle, reciprocating cylinder, flow-through cell methods. It also covers alternative dissolution testing methods like rotating bottle, peristalsis and Franz diffusion cell methods. Finally, the document discusses problems of variable control in dissolution testing and provides an overview of in vitro-in vivo correlation (IVIVC