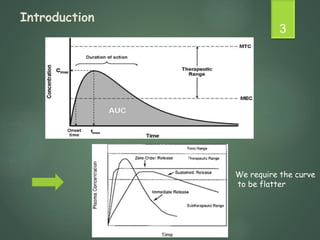
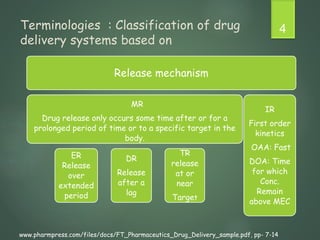
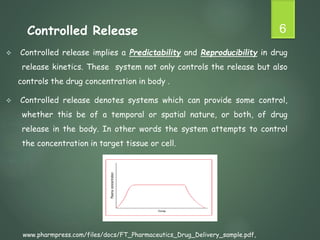
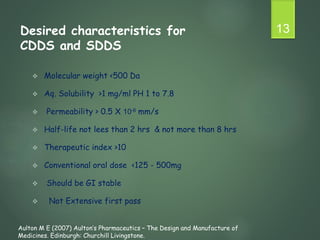
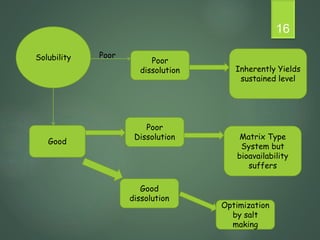
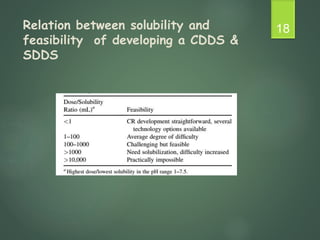
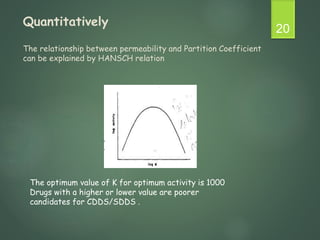
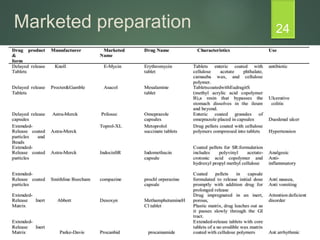
The document discusses factors that influence the design of controlled/sustained release drug products. It defines key terms like modified release, extended release, controlled release and sustained release. The main physicochemical drug properties that influence product design are aqueous solubility, partition coefficient, protein binding, drug stability, dose size and ionization constant. Controlled delivery systems aim to sustain drug action at a predetermined rate and localize drug action. The design must consider drug properties, route of administration, disease and patient factors.

















![Case Study 26
Sandberg et. al. Eur J Clin Pharmacol (1988) 33 [Suppl]: S3-S7](https://image.slidesharecdn.com/rphysicochemicalfactorsforcdds-220429060123/85/Physicochemical-factors-for-CDDS-pptx-26-320.jpg)
