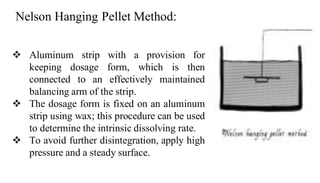
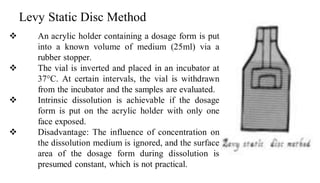
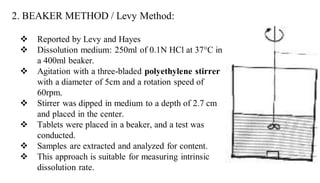
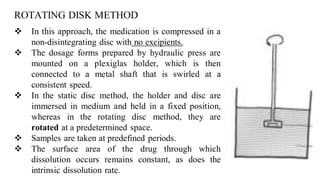
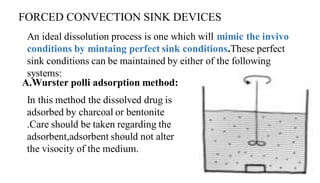
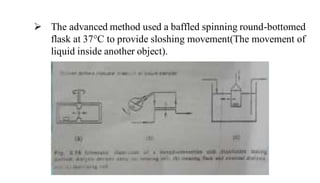
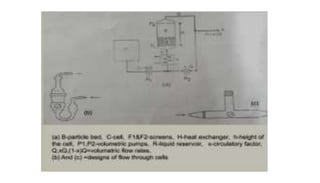
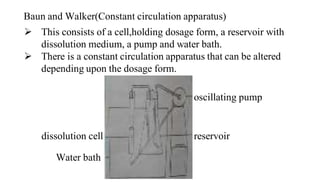
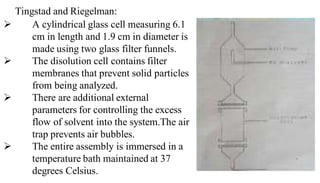
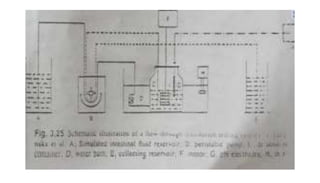
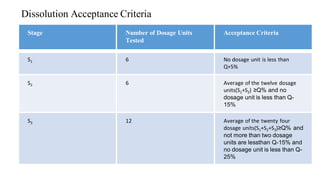
The document discusses the dissolution of drugs, outlining various apparatuses used for dissolution testing, including closed and open compartment types, along with natural and forced convection methods. It also presents specific techniques like the Klein solvmeter method, Nelson hanging pellet method, and rotating disk method, detailing their principles and limitations. Additionally, the document covers the importance of dissolution rate in drug product specifications and acceptance criteria as per regulatory guidelines.