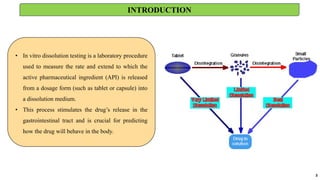
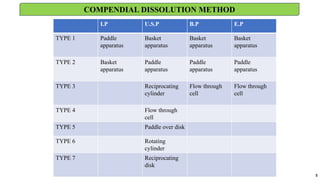
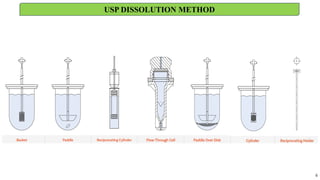
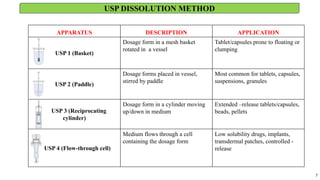
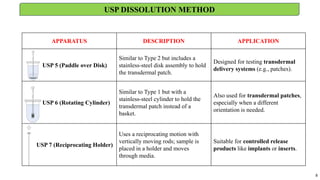
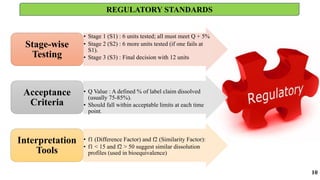
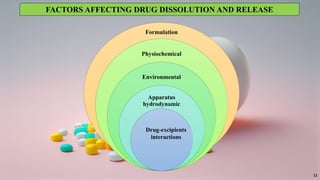
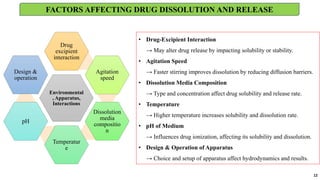
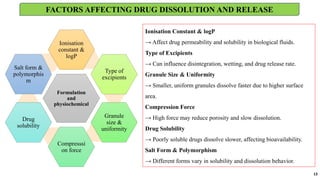
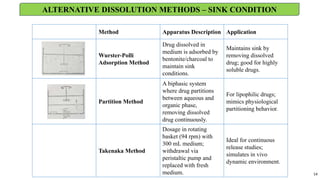
in vitro: dissolution and drug release testing, compendial methods of dissolution, alternative methods of dissolution testing, meeting dissolution requirements, problems of variable control in dissolution testing performance of drug products. In vitro-in vivo correlation, dissolution profile comparisons, drug.