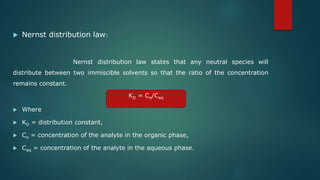
The document discusses various extraction methods for drugs from biological matrices, including techniques such as liquid-liquid extraction, solid phase extraction, and protein precipitation. It outlines the principles of each method, focusing on how proteins can be made insoluble to facilitate extraction, along with advantages and disadvantages of these techniques. Overall, it provides a comprehensive overview of the extraction processes, including reagent usage and sample preparation methods.