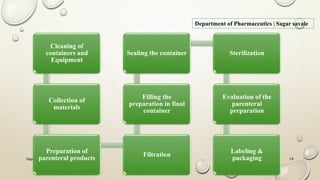
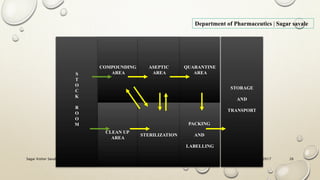
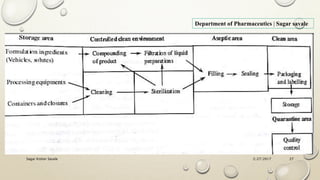
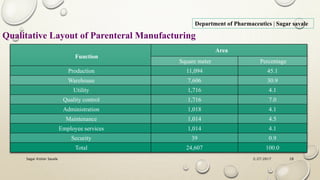
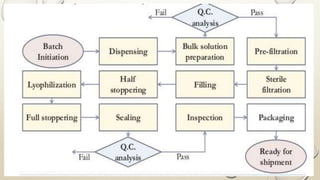
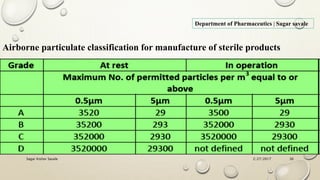
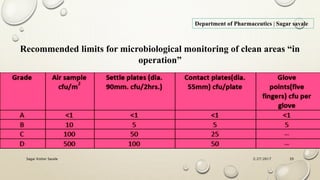
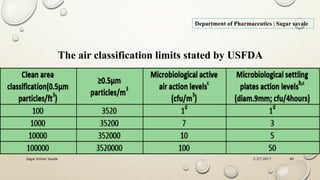
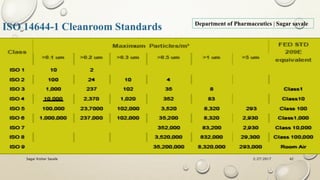
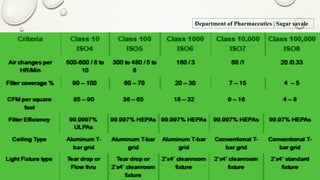
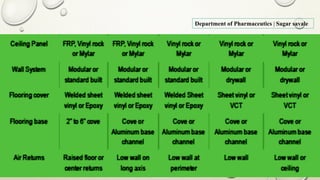
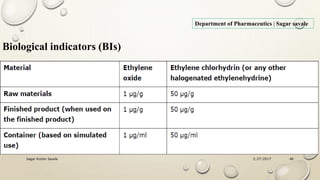
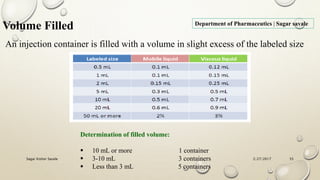
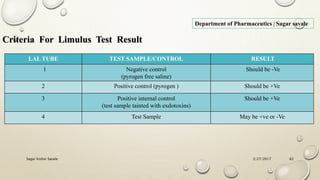
The document outlines the parenteral production department's processes, advantages, and disadvantages of parenteral administration, which bypasses the oral route of medication. It details types of parenteral preparations, requirements for successful manufacturing, environmental controls, quality assurance, and testing methods involved in ensuring sterility and safety. Additionally, it discusses different injection types and their respective clinical uses, emphasizing the importance of maintaining sterile conditions during the preparation and manufacturing process.
![Parenteral Production
Department of Pharmaceutics | Sagar savale
Mr. Sagar Kishor Savale
[Department of Pharmaceutics]
Mobile No. +91 9960885333
Email: avengersagar16@gmail.com
2/27/2017Sagar Kishor Savale 1](https://image.slidesharecdn.com/parenteralproduction-170227124241/75/Parenteral-production-1-2048.jpg)