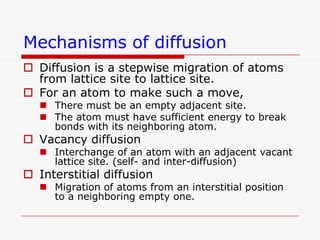
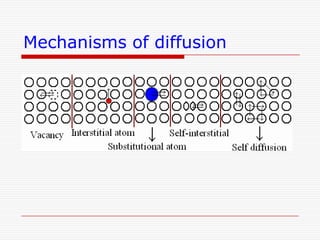
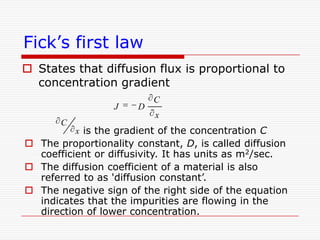
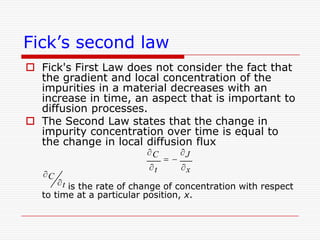
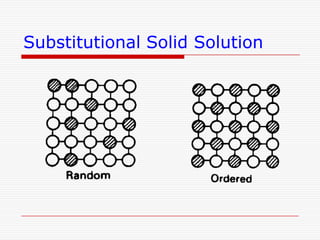
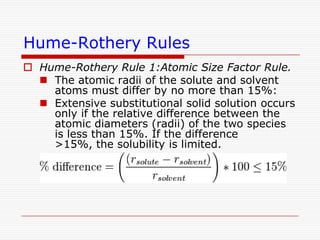
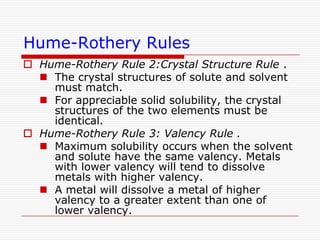
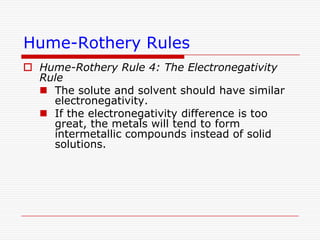
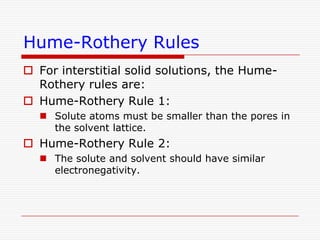
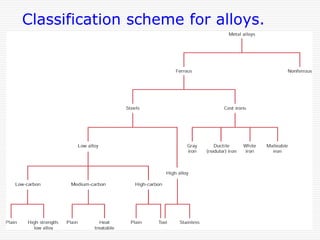
This document discusses diffusion and alloys in materials science. It defines diffusion as the movement of atoms in solids and describes mechanisms like vacancy and interstitial diffusion. Fick's laws of diffusion relating flux and concentration gradients are also covered. The document then discusses alloys, defining them as mixtures of elements and describing types like substitutional and interstitial solid solutions as well as intermetallic compounds. Rules for solid solubility like the Hume-Rothery rules are summarized. Superalloys are mentioned as high strength alloys that retain properties at high temperatures through mechanisms like grain boundary control.