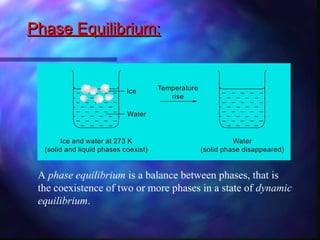
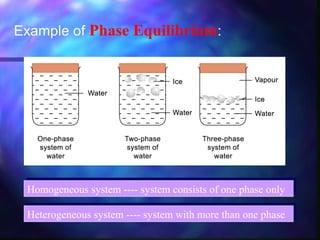
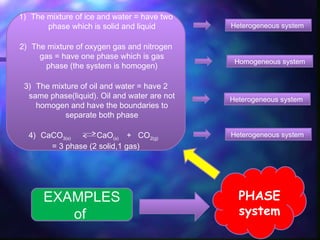
The document discusses phase equilibrium, which refers to the balance between phases in a system. There are two types of equilibrium: physical and chemical. Physical equilibrium occurs between different phases of the same substance, like liquid-gas equilibrium, while chemical equilibrium involves the forward and reverse reactions between reactants and products occurring at equal rates. An example of phase equilibrium is the mixture of ice and water, which contains solid and liquid phases in a state of dynamic equilibrium.