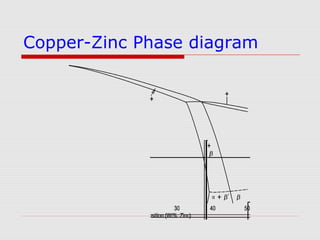
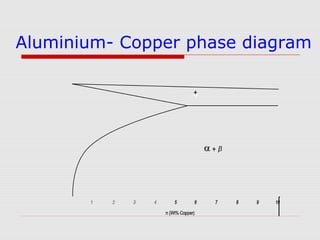
This document provides an overview of various non-ferrous alloys including copper alloys like brass and bronze, aluminium alloys like duralumin and silumin, titanium alloys like Ti-6Al-4V, magnesium alloys and their properties and applications. It discusses the alloying elements, strengthening mechanisms, microstructure and common types of each alloy. Key alloys and their uses in various industries are also summarized.