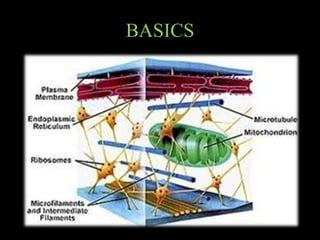
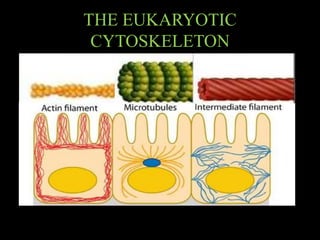
The document discusses the cytoskeleton, which is composed of microfilaments, intermediate filaments, and microtubules. Microfilaments are composed of actin and are involved in cell motility and structure. Intermediate filaments provide mechanical strength and support cellular structures. Microtubules are composed of tubulin and are involved in maintaining cell shape and intracellular transport. The cytoskeleton is a dynamic network that maintains cell structure and enables various cell functions and movements.