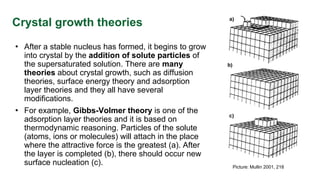
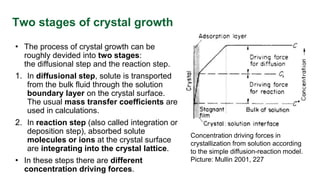
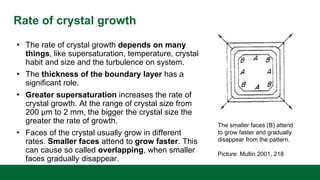
This document discusses crystal growth theories and processes. It describes how crystals form stable nuclei and then grow through the addition of solute particles. The growth process involves two main stages: a diffusional step where solute is transported to the crystal surface, and a reaction step where molecules integrate into the crystal lattice. The rate of crystal growth depends on factors like supersaturation, temperature, and crystal size and habit. Controling the growth rate can influence crystal purity and size distribution.