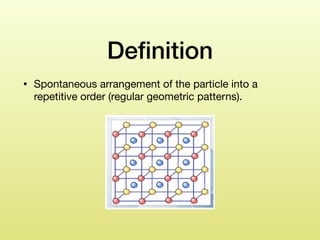
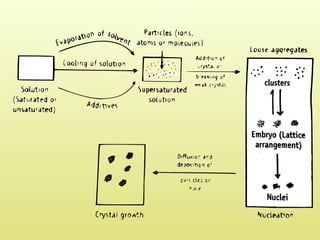
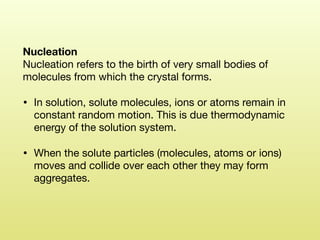
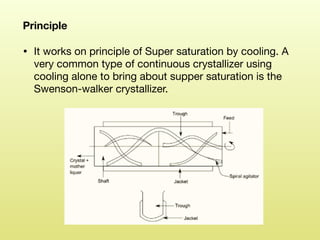
The document discusses crystallization, defining it as a spontaneous arrangement of particles into regular geometric patterns and outlining its objectives, including drug purification and improved stability. It details the processes of supersaturation, nucleation, and crystal growth, and emphasizes the industrial importance of crystallization for obtaining pure chemical substances. Additionally, it covers the prevention of caking and introduces the Swenson Walker crystallizer, highlighting its continuous operation and benefits.