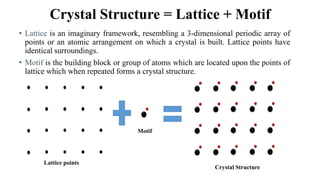
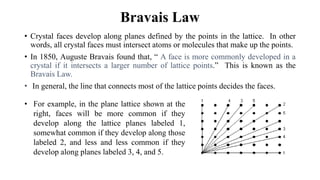
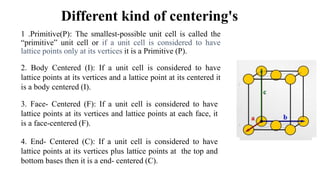
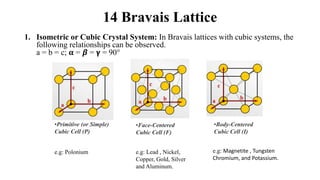
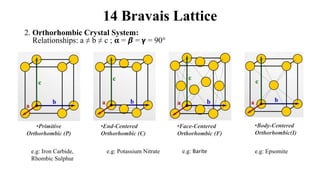
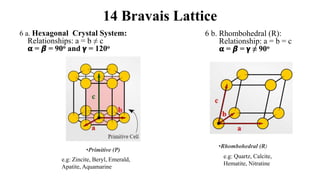
The document discusses Bravais lattices, which are the 14 possible arrangements of points in a crystal structure that satisfy translational symmetry. A Bravais lattice is composed of a motif that is repeated by translations to form the crystal structure. There are six crystal systems that the 14 Bravais lattices fall into based on their unit cell dimensions and angles. The document provides examples of common crystal structures that use each of the different Bravais lattice types.