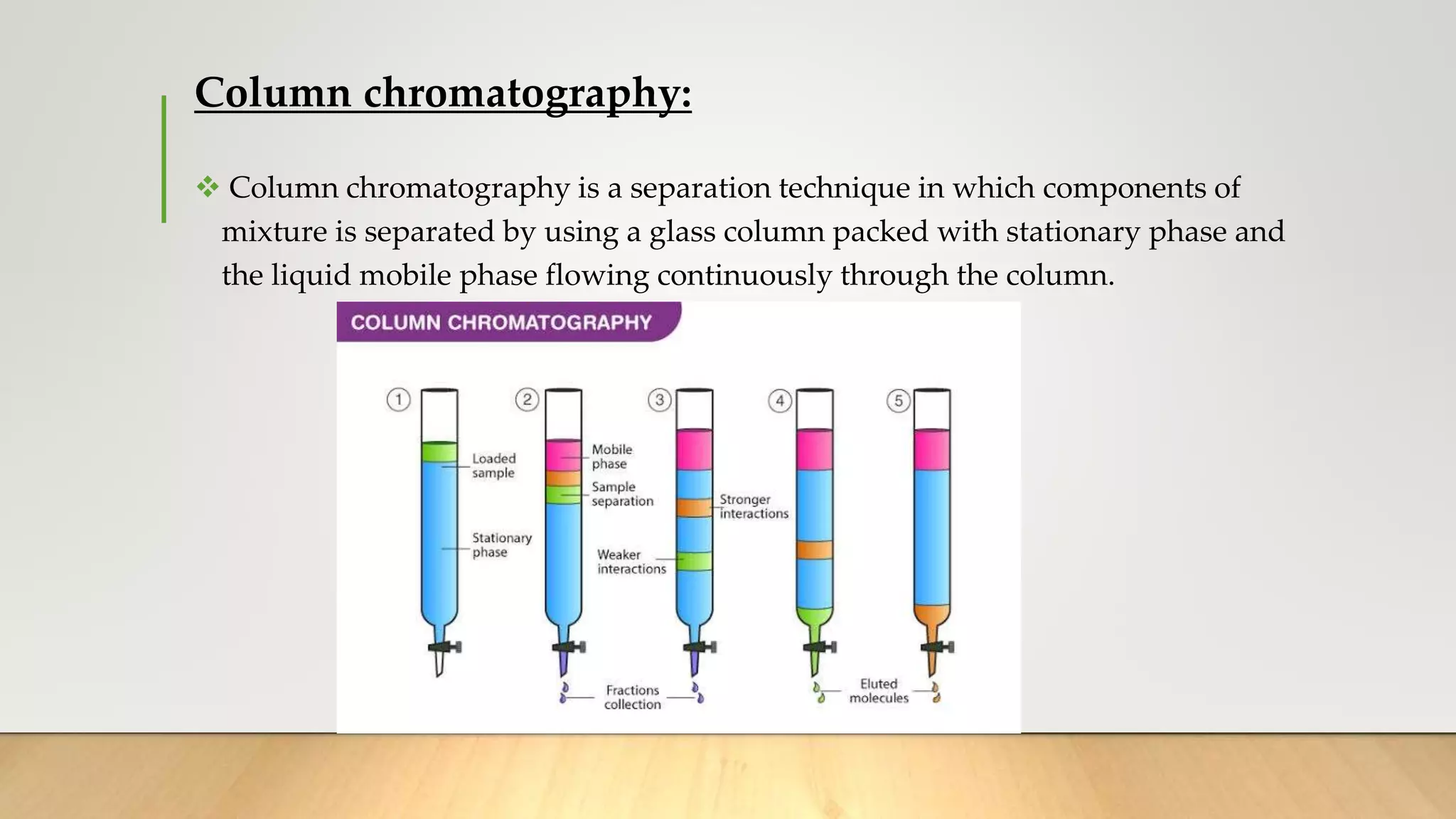
The document presents an overview of column chromatography, including its definition, history, principles, classifications, advantages, disadvantages, and applications. It explains that column chromatography separates components of a mixture using a stationary phase and a mobile phase, based on their affinities. Notable applications include the purification of compounds and the isolation of active constituents from biological fluids.